4.02 Quiz: Measuring The Flow Of Heat
Onlines
Mar 17, 2025 · 6 min read
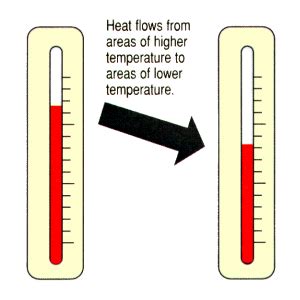
Table of Contents
4.02 Quiz: Mastering the Measurement of Heat Flow
Understanding heat flow is fundamental to numerous scientific disciplines, from thermodynamics and engineering to meteorology and climatology. This comprehensive guide delves into the core concepts of measuring heat flow, providing a detailed explanation suitable for students preparing for a 4.02 quiz or anyone seeking a deeper understanding of this crucial scientific principle. We'll cover key terms, methods, applications, and troubleshooting common misconceptions.
What is Heat Flow?
Heat flow, or heat transfer, refers to the movement of thermal energy from a region of higher temperature to a region of lower temperature. This transfer continues until thermal equilibrium is reached, meaning both regions are at the same temperature. This process is governed by the fundamental laws of thermodynamics. Understanding the rate of this heat transfer is crucial for many applications.
Three Mechanisms of Heat Transfer
Heat transfer primarily occurs through three mechanisms:
-
Conduction: This involves the transfer of heat through direct contact between molecules. Materials with high thermal conductivity, like metals, transfer heat efficiently, while insulators, such as wood or air, transfer heat poorly. Think of touching a hot stove – the heat is conducted directly to your hand.
-
Convection: This involves the transfer of heat through the movement of fluids (liquids or gases). Warmer, less dense fluid rises, while cooler, denser fluid sinks, creating a convection current. Examples include boiling water or the formation of weather patterns.
-
Radiation: This involves the transfer of heat through electromagnetic waves. No medium is required for radiation; it can occur in a vacuum. The sun's heat reaches Earth through radiation.
Measuring Heat Flow: Key Concepts and Techniques
Accurately measuring heat flow requires understanding several key concepts and utilizing appropriate techniques.
1. Heat Flux: The Rate of Heat Transfer
Heat flux (q) is defined as the rate of heat transfer per unit area. It is typically measured in Watts per square meter (W/m²). A higher heat flux indicates a faster rate of heat transfer. The formula for heat flux is often expressed as:
q = Q / (A * t)
Where:
- q is the heat flux (W/m²)
- Q is the amount of heat transferred (Joules, J)
- A is the area through which the heat is transferred (m²)
- t is the time over which the heat is transferred (seconds, s)
2. Thermal Conductivity (k): A Material's Ability to Conduct Heat
Thermal conductivity (k) is a material property that quantifies its ability to conduct heat. Materials with high thermal conductivity (e.g., copper, aluminum) transfer heat quickly, while materials with low thermal conductivity (e.g., wood, plastic) are good insulators. The SI unit for thermal conductivity is Watts per meter-kelvin (W/m·K).
3. Temperature Gradient: The Driving Force of Heat Transfer
The temperature gradient is the change in temperature over a distance. A steeper temperature gradient leads to a higher rate of heat transfer. The larger the difference in temperature between two points, and the shorter the distance between them, the greater the heat flux.
4. Methods for Measuring Heat Flux
Several methods exist for measuring heat flow, each with its own advantages and disadvantages:
-
Thermometers and Temperature Sensors: Measuring the temperature difference across a material over time allows for calculation of heat flux using the appropriate formula, considering the material's thermal conductivity and geometry. This method is suitable for simple experiments.
-
Heat Flux Meters (or Heat Flux Sensors): These devices directly measure heat flux by employing a sensor that detects the temperature difference across a thin, thermally conductive material. They are more accurate and efficient than relying solely on temperature measurements.
-
Differential Scanning Calorimetry (DSC): DSC is a sophisticated technique used to measure the heat flow associated with phase transitions (e.g., melting, crystallization) or chemical reactions. It provides detailed information about the heat transfer processes occurring during these events.
Applications of Heat Flow Measurement
Measuring heat flow has numerous applications across diverse fields:
-
Building Design and Energy Efficiency: Heat flow measurements are crucial for designing energy-efficient buildings. Insulation materials are tested to determine their effectiveness in minimizing heat loss or gain. This impacts heating and cooling costs significantly.
-
Manufacturing Processes: Precise control of heat flow is vital in various manufacturing processes, including materials processing, semiconductor fabrication, and food processing. Monitoring and managing heat flow ensures product quality and consistency.
-
Medical Applications: Understanding heat transfer is crucial in medical technologies such as thermal imaging, hyperthermia cancer treatment, and cryosurgery.
-
Environmental Science: Measuring heat flow helps in understanding climate change, analyzing heat transfer in the Earth's atmosphere and oceans, and studying the impact of human activities on the environment.
Common Misconceptions about Heat Flow
Several common misconceptions can hinder a thorough understanding of heat flow:
-
Heat is a substance: Heat is not a substance that flows like a liquid; it's a form of energy transfer.
-
Conduction only occurs in solids: While conduction is most efficient in solids, it can also occur in liquids and gases, albeit less efficiently.
-
Radiation requires a medium: Radiation does not require a medium to propagate; it can travel through a vacuum.
-
Heat always flows from hot to cold: This is generally true, but exceptions can occur in specific scenarios involving active heat pumps or other systems that use energy to reverse the natural direction of heat flow.
Troubleshooting Measurement Issues
Challenges in measuring heat flow often arise from:
-
Inaccurate temperature measurements: Ensuring accurate temperature measurements is crucial. Calibration of thermometers and sensors is vital.
-
Poor thermal contact: Insufficient contact between the sensor and the material being measured can lead to inaccurate readings. The use of thermal paste or other interface materials can improve contact.
-
Heat losses to the surroundings: Heat loss to the surrounding environment can affect the accuracy of measurements. Proper insulation and environmental control are necessary to minimize such losses.
-
Non-uniform heat flow: If the heat flow is not uniform across the surface being measured, the readings may not represent the overall heat flux accurately.
Preparing for your 4.02 Quiz: Practice Problems and Tips
Thorough preparation is key to success on your 4.02 quiz. Practice solving various problems involving heat flux calculations and understanding the different methods of heat transfer. Focus on understanding the concepts rather than rote memorization of formulas.
Here are some example problems:
Problem 1: A heat flux meter measures a heat flux of 200 W/m² through a 2 cm thick wall with an area of 1 m². If the temperature difference across the wall is 20°C, what is the wall's thermal conductivity?
Problem 2: A 100 W light bulb is placed in a room. Assuming all the light bulb's energy is converted into heat and radiates uniformly in all directions, what is the heat flux at a distance of 1 meter from the bulb? (Hint: Consider the surface area of a sphere).
Tips for Success:
- Review the definitions of key terms: Ensure you understand the meanings of terms like heat flux, thermal conductivity, and temperature gradient.
- Practice calculations: Work through numerous practice problems to hone your calculation skills.
- Understand the different methods of heat transfer: Be able to distinguish between conduction, convection, and radiation.
- Identify potential sources of error in measurements: Be aware of factors that can affect the accuracy of heat flow measurements.
By mastering the concepts discussed in this guide and practicing diligently, you'll be well-prepared to excel on your 4.02 quiz and gain a strong understanding of the fascinating world of heat flow. Remember to always check your work and understand the reasoning behind your answers. Good luck!
Latest Posts
Latest Posts
-
Which Of The Following Is Not Part Of Feminist Psychology
Mar 17, 2025
-
Which Puritan Value Most Influenced The Emerging Neoclassical Style
Mar 17, 2025
-
Things Fall Apart Chapter 4 Summary
Mar 17, 2025
-
One Issue Dual Language Programs Face Is
Mar 17, 2025
-
Quotes From Where The Red Fern Grows
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about 4.02 Quiz: Measuring The Flow Of Heat . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
