Atoms Ions And Isotopes Worksheet Answers
Onlines
Mar 16, 2025 · 6 min read
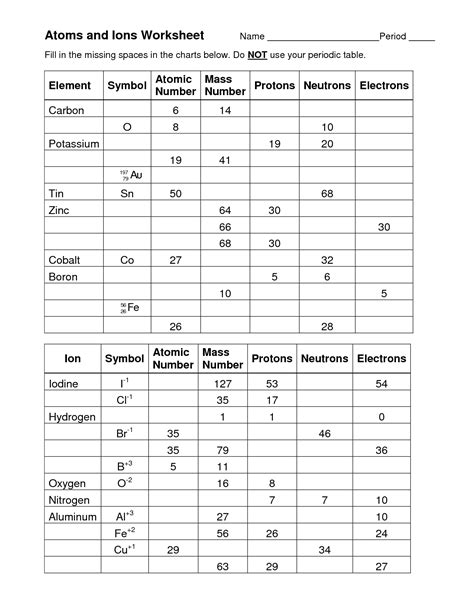
Table of Contents
Atoms, Ions, and Isotopes: A Comprehensive Worksheet and Answer Guide
Understanding atoms, ions, and isotopes is fundamental to grasping the basics of chemistry. This comprehensive guide will walk you through the key concepts, providing explanations and answers to common worksheet questions. We'll explore the structure of atoms, the formation of ions, and the variations found in isotopes, equipping you with a solid foundation in atomic theory.
What is an Atom?
At its core, an atom is the basic building block of matter. It's the smallest unit of an element that retains the chemical properties of that element. Think of atoms like tiny LEGO bricks – each brick (atom) is unique, but you can combine them in countless ways to build larger structures (molecules).
Key Components of an Atom:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons determines the element's atomic number and defines its identity.
- Neutrons: Neutral particles (no charge) also residing in the atom's nucleus. Along with protons, they contribute to the atom's mass number.
- Electrons: Negatively charged particles orbiting the nucleus in electron shells or energy levels. The number of electrons usually equals the number of protons in a neutral atom.
Ions: Charged Atoms
An ion is an atom or molecule that has gained or lost one or more electrons, resulting in a net electric charge. This charge imbalance is what distinguishes an ion from a neutral atom.
Types of Ions:
- Cations: Positively charged ions. These form when an atom loses electrons. Metals tend to form cations. For example, a sodium atom (Na) can lose one electron to become a sodium ion (Na⁺).
- Anions: Negatively charged ions. These form when an atom gains electrons. Nonmetals tend to form anions. For example, a chlorine atom (Cl) can gain one electron to become a chloride ion (Cl⁻).
Isotopes: Variations of an Element
Isotopes are atoms of the same element that have the same number of protons but a different number of neutrons. This means they have the same atomic number but a different mass number. The difference in neutron number doesn't change the element's chemical properties significantly, but it can affect its physical properties, such as radioactivity.
Understanding Atomic Number and Mass Number:
- Atomic Number (Z): The number of protons in an atom's nucleus. This uniquely identifies the element.
- Mass Number (A): The total number of protons and neutrons in an atom's nucleus.
Worksheet Questions and Answers:
Let's address some common questions found in atoms, ions, and isotopes worksheets. Remember, the specific questions will vary, but the underlying principles remain the same.
Question 1: What are the three subatomic particles that make up an atom? Describe their charge and location.
Answer: The three subatomic particles are:
- Protons: Positive charge (+1), located in the nucleus.
- Neutrons: Neutral charge (0), located in the nucleus.
- Electrons: Negative charge (-1), located in electron shells or energy levels surrounding the nucleus.
Question 2: Explain the difference between atomic number and mass number.
Answer:
- Atomic number (Z): Represents the number of protons in an atom's nucleus. It uniquely identifies the element.
- Mass number (A): Represents the total number of protons and neutrons in an atom's nucleus. It indicates the atom's mass.
Question 3: What is an ion? Give an example of a cation and an anion.
Answer: An ion is an atom or molecule that carries a net electric charge due to the gain or loss of electrons.
- Cation (positive ion): Example: Sodium ion (Na⁺), formed when a sodium atom loses one electron.
- Anion (negative ion): Example: Chloride ion (Cl⁻), formed when a chlorine atom gains one electron.
Question 4: Define isotopes. Explain how isotopes of the same element differ.
Answer: Isotopes are atoms of the same element that have the same number of protons (atomic number) but a different number of neutrons. This leads to a different mass number. They have the same chemical properties but may have slightly different physical properties.
Question 5: An atom has 17 protons, 18 neutrons, and 17 electrons. Identify the element, its atomic number, and its mass number.
Answer:
- Element: Chlorine (Cl) – the atomic number 17 identifies it as chlorine.
- Atomic number (Z): 17 (number of protons)
- Mass number (A): 35 (number of protons + neutrons = 17 + 18)
Question 6: Carbon-12 and Carbon-14 are isotopes of carbon. Explain how they differ.
Answer: Both Carbon-12 and Carbon-14 have 6 protons (this defines them as carbon). However, Carbon-12 has 6 neutrons (12 - 6 = 6), while Carbon-14 has 8 neutrons (14 - 6 = 8). The difference in neutron number makes Carbon-14 radioactive.
Question 7: Draw a simple diagram of an atom, labeling its key components.
Answer: (A simple drawing would show a central nucleus containing protons and neutrons, with electrons orbiting in shells around the nucleus.) You would label the nucleus, protons (+), neutrons (0), and electrons (-).
Question 8: Explain how ions are formed.
Answer: Ions are formed when atoms gain or lose electrons to achieve a more stable electron configuration, typically a full outer electron shell. Metals tend to lose electrons (forming cations), and nonmetals tend to gain electrons (forming anions).
Question 9: What is the significance of the electronic configuration of an atom in determining its chemical behavior?
Answer: The arrangement of electrons in an atom's energy levels (electronic configuration) determines its chemical behavior. Atoms tend to react in ways that allow them to achieve a stable, full outer electron shell. This drive for stability governs how atoms bond with each other, influencing the chemical properties of elements and compounds.
Question 10: How does the number of neutrons affect the stability of an atom?
Answer: The ratio of protons to neutrons significantly impacts an atom's stability. Certain ratios are more stable than others. If this ratio is significantly off balance (too many or too few neutrons compared to protons), the atom may be radioactive and undergo decay to achieve a more stable configuration.
Advanced Concepts and Further Exploration:
This worksheet and answer guide provide a foundational understanding of atoms, ions, and isotopes. However, there are further aspects you can explore:
- Radioactive Isotopes: Delve deeper into radioactive decay, half-life, and applications of radioactive isotopes in medicine and other fields.
- Mass Spectrometry: Learn how this technique is used to determine the relative abundance of isotopes.
- Ionic Bonding and Covalent Bonding: Explore how atoms combine to form molecules through different types of chemical bonds.
- Isotopic Abundance and Average Atomic Mass: Understand how to calculate the average atomic mass of an element based on the relative abundance of its isotopes.
By continuing to explore these topics, you can build upon the fundamental concepts presented here and develop a more comprehensive understanding of atomic structure and its implications in the world of chemistry. Remember to practice solving various problems to solidify your understanding. This will ensure you are well-prepared for more advanced chemistry concepts.
Latest Posts
Latest Posts
-
Elige La Palabra Adecuada Para Completar Las Oraciones Comparativas
Mar 17, 2025
-
You Seem Pleased To Have Successfully Whored Yourself Manacled
Mar 17, 2025
-
Muchas Ninas Jovenes Han Estado A Dieta Terrible
Mar 17, 2025
-
Examples Of Public Data Collected By Law From Physicians Include
Mar 17, 2025
-
Alcohol And Its Effects On The Body Worksheet Answers
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Atoms Ions And Isotopes Worksheet Answers . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
