Average Atomic Mass Gizmo Answer Key
Onlines
Mar 21, 2025 · 5 min read
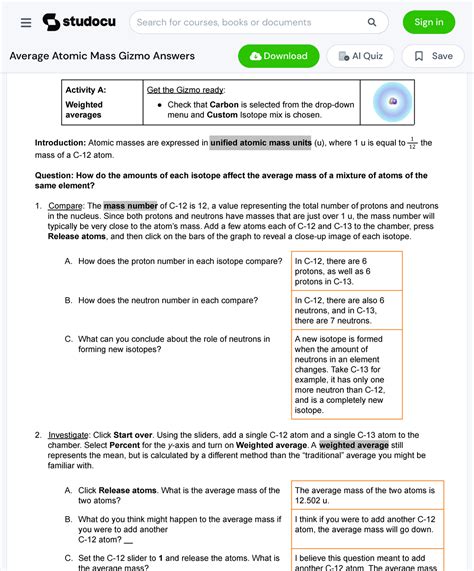
Table of Contents
Average Atomic Mass Gizmo Answer Key: A Comprehensive Guide
The Average Atomic Mass Gizmo is a valuable tool for students learning about isotopes and the weighted average calculation of atomic mass. This article serves as a comprehensive guide, providing not only the answers but also a detailed explanation of the concepts involved. Understanding these concepts is crucial for mastering chemistry, so let's delve into the intricacies of average atomic mass.
Understanding Isotopes and Atomic Mass
Before we jump into the Gizmo's specifics, let's establish a strong foundation. Atoms of the same element can have different numbers of neutrons, leading to variations called isotopes. Each isotope has a different mass number, which is the sum of protons and neutrons. The atomic number, however, remains constant, representing the number of protons and defining the element.
For example: Carbon-12 and Carbon-14 are isotopes of carbon. Both have 6 protons (atomic number 6), but Carbon-12 has 6 neutrons (mass number 12), while Carbon-14 has 8 neutrons (mass number 14).
Atomic mass, often referred to as atomic weight, is the weighted average mass of all the isotopes of an element. It's not the mass of a single atom but rather a representation of the average mass considering the abundance of each isotope. This is crucial because elements in nature exist as a mixture of isotopes, not just one single type.
The Average Atomic Mass Gizmo: A Step-by-Step Walkthrough
The Gizmo typically presents you with different isotopes of an element, their masses, and their relative abundances. Your task is to calculate the average atomic mass using this information. Here's a breakdown of the process:
Step 1: Identify the Isotopes and their Data
The Gizmo will usually provide a table or similar visual representation listing each isotope of the element, its mass number (or mass in atomic mass units, amu), and its percent abundance. Carefully note down these values. Accuracy in this step is critical for the subsequent calculations.
Step 2: Convert Percentages to Decimal Form
Percent abundances are given as percentages (%). To use them in the weighted average calculation, you must convert them into decimal form by dividing by 100. For example, 75% becomes 0.75.
Step 3: Perform the Weighted Average Calculation
The weighted average calculation for atomic mass involves multiplying the mass of each isotope by its decimal abundance, and then summing these products. The formula can be expressed as:
Average Atomic Mass = (Mass of Isotope 1 × Abundance of Isotope 1) + (Mass of Isotope 2 × Abundance of Isotope 2) + ...
This process continues for all isotopes present.
Step 4: Analyze and Interpret the Results
Compare your calculated average atomic mass with the value provided by the Gizmo (or found on a periodic table). Minor discrepancies might arise due to rounding errors. However, the values should be quite close. This comparison reinforces your understanding of the calculation process and the concept of weighted averages.
Example Calculation: Average Atomic Mass of Boron
Let's work through a hypothetical example using the Gizmo's structure. Assume the Gizmo provides the following data for Boron:
- Boron-10: Mass = 10 amu, Abundance = 20%
- Boron-11: Mass = 11 amu, Abundance = 80%
Step 1 & 2: Data Collection and Percentage Conversion
- Boron-10: Mass = 10 amu, Abundance = 0.20
- Boron-11: Mass = 11 amu, Abundance = 0.80
Step 3: Weighted Average Calculation
Average Atomic Mass = (10 amu × 0.20) + (11 amu × 0.80) = 2 amu + 8.8 amu = 10.8 amu
Step 4: Result Analysis
The calculated average atomic mass of Boron is 10.8 amu. This value closely aligns with the accepted average atomic mass of Boron found on the periodic table.
Advanced Concepts and Troubleshooting
The Gizmo might introduce variations or complexities. Here are some scenarios and how to tackle them:
Dealing with More Isotopes
If the Gizmo includes more than two isotopes, simply extend the weighted average calculation formula. Continue multiplying each isotope's mass by its decimal abundance and summing the results. The principle remains the same, regardless of the number of isotopes involved.
Isotopic Abundance in Different Sources
The isotopic abundance of an element can vary slightly depending on the source. This variation is due to natural processes and shouldn't cause significant discrepancies in your calculated average atomic mass compared to values found in textbooks or online.
Understanding Significant Figures
Pay attention to the number of significant figures given in the data provided by the Gizmo. Your final answer should reflect the correct number of significant figures, based on the least precise measurement provided. This ensures accuracy and precision in your calculations.
Using Scientific Notation
For isotopes with very high or very low abundances, the Gizmo might present the data in scientific notation. Remember to convert these values to decimal form before performing the weighted average calculation.
Beyond the Gizmo: Real-World Applications
Understanding average atomic mass is essential in numerous scientific fields. It's crucial in:
- Chemistry: Determining the molar mass of compounds, which is critical for stoichiometric calculations.
- Nuclear Physics: Studying nuclear reactions and radioactive decay processes involving different isotopes.
- Mass Spectrometry: Analyzing the isotopic composition of samples and identifying unknown substances.
- Geochemistry: Studying the isotopic ratios of elements in geological samples to understand Earth's processes.
Conclusion: Mastering the Average Atomic Mass
The Average Atomic Mass Gizmo provides a valuable interactive platform for mastering a fundamental concept in chemistry. By understanding the principles of isotopes, weighted averages, and applying the correct calculation methods, students can develop a strong foundation in atomic structure and its applications. This guide has attempted to provide a detailed and comprehensive walkthrough, aiding in successful completion of the Gizmo activities and fostering a deeper understanding of average atomic mass calculations. Remember to always practice and utilize the concepts learned to reinforce your knowledge and achieve a deeper understanding of chemistry.
Latest Posts
Latest Posts
-
One Problem With Conducting A Social Audit Is
Mar 22, 2025
-
Answer Each Question Affirmatively Using The Correct Possessive Adjective
Mar 22, 2025
-
Fire Doors Also Called Smoke Barrier Doors Should
Mar 22, 2025
-
The Suffix Denoting An Instrument Used To Measure Is
Mar 22, 2025
-
Summary Of The Giver Chapter 17
Mar 22, 2025
Related Post
Thank you for visiting our website which covers about Average Atomic Mass Gizmo Answer Key . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
