Calories Evolved Per Mole Of H
Onlines
Mar 12, 2025 · 5 min read
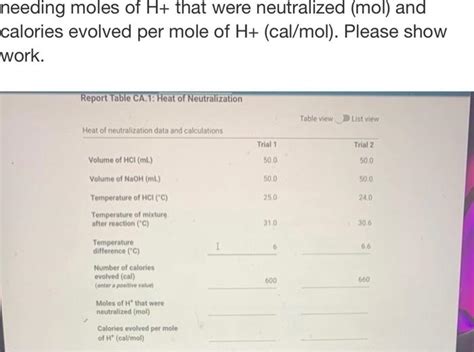
Table of Contents
Calories Evolved Per Mole of H: A Deep Dive into Thermochemistry
Understanding the energy changes associated with chemical reactions is crucial in various fields, from chemistry and biochemistry to engineering and environmental science. One fundamental concept in this area is the heat of reaction, specifically the calories evolved (or absorbed) per mole of a particular substance involved. This article delves into the intricacies of calculating and understanding the calories evolved per mole of hydrogen (H), focusing on different reaction scenarios and the underlying thermodynamic principles.
Understanding Enthalpy and its Relation to Heat of Reaction
Before we dive into hydrogen-specific calculations, let's establish a firm grasp of fundamental thermodynamic concepts. The enthalpy (H) of a system represents its total heat content at constant pressure. Changes in enthalpy (ΔH) reflect the heat exchanged between a system and its surroundings during a process at constant pressure. A negative ΔH indicates an exothermic reaction—heat is released to the surroundings—while a positive ΔH signifies an endothermic reaction, where heat is absorbed from the surroundings.
The heat of reaction (also known as the enthalpy change of reaction) is the enthalpy change that occurs during a chemical reaction. It's usually expressed in kilojoules per mole (kJ/mol) or kilocalories per mole (kcal/mol). The number of calories evolved (or absorbed) per mole of H will depend entirely on the specific reaction being considered. There's no single universal value.
Calculating Calories Evolved Per Mole of H in Combustion Reactions
Hydrogen's combustion reaction is a highly exothermic process, producing a significant amount of heat. The balanced chemical equation is:
2H₂(g) + O₂(g) → 2H₂O(l)
This reaction releases a substantial amount of energy. To determine the calories evolved per mole of H, we need to consider the standard enthalpy change of combustion (ΔH°comb) for hydrogen. This value is typically found in thermodynamic data tables. The standard enthalpy change of combustion for hydrogen is approximately -285.8 kJ/mol for the formation of liquid water.
Calculations:
Since 2 moles of H₂ are involved in the reaction, and each mole of H₂ contains 2 moles of H, the enthalpy change per mole of H is:
(-285.8 kJ/mol H₂) / (2 mol H/mol H₂) = -142.9 kJ/mol H
To convert this to kilocalories:
-142.9 kJ/mol H * (1 kcal/4.184 kJ) ≈ -34.15 kcal/mol H
Therefore, approximately 34.15 kcal of heat are evolved per mole of hydrogen during its complete combustion to form liquid water. This is a significant amount of energy, highlighting hydrogen's potential as a fuel source.
Influence of Different Reaction Conditions
The calories evolved per mole of H can vary depending on several factors:
-
State of Matter: The enthalpy change depends on the physical states of the reactants and products (gas, liquid, or solid). For example, the combustion of hydrogen to form gaseous water (H₂O(g)) would yield a different ΔH compared to liquid water (H₂O(l)). The gaseous form will result in less heat being evolved.
-
Temperature and Pressure: These factors affect the kinetic energy of molecules and can influence the rate of reaction and the overall enthalpy change. Standard enthalpy changes are typically reported at standard temperature and pressure (STP: 273.15 K and 1 atm).
-
Presence of Catalysts: Catalysts can lower the activation energy of a reaction without being consumed themselves. While catalysts do not alter the overall enthalpy change of the reaction, they might affect the rate at which the reaction releases energy, appearing to affect the "calories evolved per mole" in practice, though not theoretically.
Beyond Combustion: Other Reactions Involving Hydrogen
Hydrogen participates in numerous other reactions, each with its own unique enthalpy change. Let's consider a few examples:
-
Hydrogenation Reactions: Hydrogenation involves the addition of hydrogen to unsaturated organic compounds (like alkenes or alkynes), converting double or triple bonds into single bonds. These reactions are generally exothermic, but the amount of heat evolved varies significantly depending on the specific organic molecule involved. The calculations require knowledge of the enthalpy of formation for both reactants and products.
-
Reactions with Halogens: Hydrogen reacts vigorously with halogens (F₂, Cl₂, Br₂, I₂) to form hydrogen halides (HF, HCl, HBr, HI). These reactions are also highly exothermic. The enthalpy of formation of these hydrogen halides can be used to calculate the heat evolved per mole of hydrogen involved.
-
Reactions with Metals: Certain metals, especially alkali and alkaline earth metals, react readily with hydrogen at high temperatures to form metal hydrides. These reactions are often exothermic, but again, the precise energy released depends on the specific metal involved.
Applications and Significance
Understanding the calories evolved per mole of H has numerous applications:
-
Fuel Cell Technology: Fuel cells utilize the electrochemical reaction between hydrogen and oxygen to directly generate electricity, releasing heat as a byproduct. Knowledge of the enthalpy change is crucial for designing efficient and safe fuel cells.
-
Energy Storage: Hydrogen is considered a promising energy carrier for storing renewable energy sources like solar and wind power. The energy released upon combustion reflects the efficiency of hydrogen storage.
-
Industrial Processes: Hydrogen is a vital reactant in numerous industrial processes, including the production of ammonia (Haber-Bosch process) and the refining of petroleum. Precise knowledge of the energy changes in these processes is essential for optimization and cost-effectiveness.
-
Thermodynamic Calculations and Predictions: The enthalpy change of reaction involving hydrogen is essential for performing broader thermodynamic calculations, such as equilibrium constant calculations and Gibbs Free Energy predictions, which are important for understanding the spontaneity and feasibility of chemical processes.
Conclusion
The calories evolved per mole of H is not a single, fixed value; it depends entirely on the specific chemical reaction in question. While combustion provides a highly exothermic and widely studied example, numerous other reactions involving hydrogen exhibit varied energy changes. Accurate determination of these values requires knowledge of standard enthalpies of formation, reaction conditions, and a detailed understanding of thermochemical principles. This knowledge is critical for advancing various technologies and optimizing industrial processes that rely on hydrogen's unique properties. Further exploration into the specific reaction context is crucial for precise calculations and a deeper understanding of the energy changes involved. Therefore, thorough research and precise data are essential for accurate and relevant calculations in different contexts.
Latest Posts
Latest Posts
-
In The Space That Follows Sketch The Indicated Cells
Mar 12, 2025
-
A Thousand Splendid Suns Chapter Summary
Mar 12, 2025
-
Medians And Centroids Worksheet Answers Gina Wilson
Mar 12, 2025
-
Break Lines Are Used To Show That
Mar 12, 2025
-
Who Doesdinah Lov Ein The Red Tent
Mar 12, 2025
Related Post
Thank you for visiting our website which covers about Calories Evolved Per Mole Of H . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
