Choose The Best Option For The Nucleophile Precursor To 3-hexyne
Onlines
Mar 23, 2025 · 6 min read
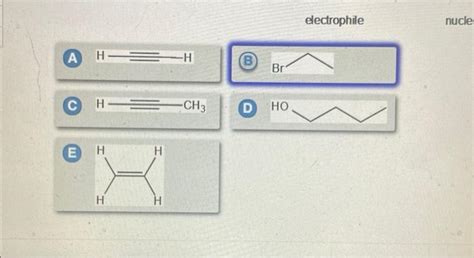
Table of Contents
Choosing the Best Nucleophile Precursor for 3-Hexyne Synthesis: A Comprehensive Guide
Synthesizing 3-hexyne requires careful consideration of the nucleophile precursor. The choice significantly impacts reaction yield, efficiency, and overall cost-effectiveness. This comprehensive guide explores various options, analyzing their strengths, weaknesses, and suitability for different synthetic strategies. We'll delve into the intricacies of nucleophilic attack on electrophilic substrates, ultimately guiding you to the optimal choice for your specific needs.
Understanding the Synthesis of 3-Hexyne
3-Hexyne, a symmetrical internal alkyne, can be synthesized through several routes, each involving a nucleophile and an electrophile. The most common methods utilize alkyl halides or sulfonates as electrophiles, reacting them with a suitable carbon nucleophile. The core reaction involves the formation of a carbon-carbon bond via nucleophilic substitution or elimination-addition mechanisms. The choice of nucleophile precursor dictates the reaction pathway and its success.
Evaluating Potential Nucleophile Precursors
Several potential nucleophile precursors can generate the necessary carbanion for 3-hexyne synthesis. Let's analyze the most prominent options:
1. Acetylide Anions from Terminal Alkynes: A Classic Approach
This approach uses a terminal alkyne as the starting material. Treatment with a strong base, such as sodium amide (NaNH2) in liquid ammonia or n-butyllithium (n-BuLi) in an aprotic solvent like tetrahydrofuran (THF), deprotonates the terminal alkyne, forming an acetylide anion. This anion acts as a powerful nucleophile.
Mechanism:
The strong base abstracts the acidic proton from the terminal alkyne, generating a negatively charged carbon atom (the acetylide anion). This anion then attacks an alkyl halide (in this case, a 1-bromopropane), undergoing an SN2 reaction to form 3-hexyne.
Advantages:
- Relatively straightforward: The reaction mechanism is well-understood and readily implemented.
- Good yields: Under optimized conditions, this method can provide good yields of 3-hexyne.
Disadvantages:
- Strong base required: The use of strong bases necessitates careful handling and anhydrous conditions to avoid unwanted side reactions.
- Competition with other reactions: The acetylide anion can participate in other reactions besides the desired SN2, lowering yield. For instance, it can undergo elimination reactions or proton abstraction.
- Substrate limitations: The alkyl halide must be relatively unhindered for the SN2 reaction to proceed efficiently. Sterically hindered alkyl halides will lead to poor yields.
2. Grignard Reagents: A Versatile Alternative
Grignard reagents (RMgX, where R is an alkyl group and X is a halide) are another viable option. They're prepared by reacting an alkyl halide with magnesium metal in an ether solvent. The resulting organomagnesium compound can then react with an electrophile.
Mechanism:
The Grignard reagent acts as a nucleophile, attacking an electrophilic carbonyl compound (in this case, a properly chosen ketone or aldehyde precursor which upon dehydration yields 3-hexyne). The subsequent dehydration step generates the alkyne. The exact precursor choice will depend on the desired regioselectivity.
Advantages:
- Versatility: Grignard reagents are versatile nucleophiles and can be used to synthesize a wide range of compounds.
- Wide availability of starting materials: Alkyl halides are readily available and relatively inexpensive.
Disadvantages:
- Sensitivity to moisture and oxygen: Grignard reagents are highly reactive and must be handled under strictly anhydrous and anaerobic conditions.
- Potential for side reactions: Grignard reagents can undergo various side reactions, reducing yield.
- Requires multiple steps: The synthesis of 3-hexyne via a Grignard reagent often requires a multi-step synthesis, including a subsequent dehydration step.
3. Organolithium Reagents: High Reactivity, High Selectivity
Organolithium reagents (RLi) share similarities with Grignard reagents but are generally more reactive. They are prepared by reacting an alkyl halide with lithium metal.
Mechanism: Similar to Grignard reagents, organolithium reagents attack an electrophilic precursor which can be subjected to elimination/dehydration.
Advantages:
- High reactivity: Organolithium reagents are more reactive than Grignard reagents, allowing for reactions with less reactive electrophiles.
- Excellent selectivity: In many cases, organolithium reagents exhibit better regio- and chemoselectivity than Grignard reagents.
Disadvantages:
- Extremely reactive: Their high reactivity necessitates extremely stringent reaction conditions and careful handling.
- Sensitivity to moisture and oxygen: Similar to Grignard reagents, they are highly sensitive to moisture and oxygen.
- Potentially explosive: Certain organolithium reagents can be explosive, particularly at higher concentrations.
4. Wittig Reaction: A Powerful Method for Alkyne Synthesis
The Wittig reaction provides a powerful alternative for forming alkenes and alkynes, offering control over the stereochemistry of the final product. It generally uses a phosphonium ylide as the nucleophile. While not directly producing an acetylide, it offers an indirect route to 3-hexyne through the formation of a precursor which can then be converted.
Mechanism: This is a multi-step process involving the formation of a phosphonium ylide, reacting it with a ketone or aldehyde, then converting the resulting alkene into an alkyne via further reactions.
Advantages:
- Control over stereochemistry: The Wittig reaction allows control over the stereochemistry of the alkene formed, offering precise control over the final alkyne.
- Versatile: It can be used to synthesize a wide range of alkynes.
Disadvantages:
- Multi-step synthesis: It's a more complex multi-step process compared to direct acetylide formation.
- Requires specialized reagents: Phosphonium ylides are not as readily available as other nucleophile precursors.
Choosing the Optimal Precursor: A Comparative Analysis
The best nucleophile precursor depends on various factors, including the scale of the synthesis, the availability of starting materials, the desired yield, and the level of experience of the chemist.
| Precursor | Advantages | Disadvantages | Suitability |
|---|---|---|---|
| Acetylide Anion | Relatively simple, good yields under optimized conditions | Requires strong bases, sensitive to side reactions, substrate limitations | Suitable for small-scale synthesis with readily available electrophiles |
| Grignard Reagent | Versatile, widely available starting materials | Sensitive to moisture and oxygen, potential for side reactions | Suitable for medium-scale synthesis, requires careful handling |
| Organolithium Reagent | High reactivity, excellent selectivity | Extremely reactive, sensitive to moisture and oxygen, potentially explosive | Best suited for experienced chemists, small scale and reactions where high selectivity is crucial |
| Wittig Reaction | Control over stereochemistry, versatile | Multi-step synthesis, requires specialized reagents | Suitable for specific stereochemical requirements, larger scale synthesis |
Practical Considerations and Optimization
Regardless of the chosen precursor, several practical considerations influence the success of 3-hexyne synthesis:
- Solvent selection: The choice of solvent significantly impacts reaction kinetics and selectivity. Aprotic solvents such as THF, diethyl ether, or DMF are commonly employed.
- Temperature control: Maintaining the appropriate reaction temperature is crucial for optimal yield and minimizing side reactions.
- Reaction time: The reaction time should be optimized to ensure complete conversion without prolonged exposure to reaction conditions, which might lead to side reactions.
- Purification: Effective purification techniques are necessary to isolate pure 3-hexyne. Techniques like distillation or chromatography are often employed.
Conclusion: Navigating the Synthesis of 3-Hexyne
The synthesis of 3-hexyne offers a valuable case study in the importance of carefully selecting the appropriate nucleophile precursor. Each option – acetylide anions, Grignard reagents, organolithium reagents, and the Wittig reaction – presents unique advantages and limitations. The optimal choice depends on your specific needs, resources, and expertise. By carefully considering the factors discussed above, researchers can successfully synthesize 3-hexyne with high yield and efficiency, contributing to the advancement of organic chemistry and related fields. This detailed analysis equips you to make informed decisions, ensuring a smooth and productive synthetic pathway. Remember, always prioritize safety and adhere to proper laboratory procedures.
Latest Posts
Latest Posts
-
A Nosotros Perder Las Llaves Del Auto
Mar 25, 2025
-
Gizmo Student Exploration Cell Division Answers
Mar 25, 2025
-
When You Are Tired Your Shrinks
Mar 25, 2025
-
How Many Chapters In Jane Eyre
Mar 25, 2025
-
Student Exploration Seasons Earth Moon And Sun
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Choose The Best Option For The Nucleophile Precursor To 3-hexyne . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
