Choose The Thermodynamic Product Formed During The Reaction Depicted Below
Onlines
Mar 20, 2025 · 5 min read
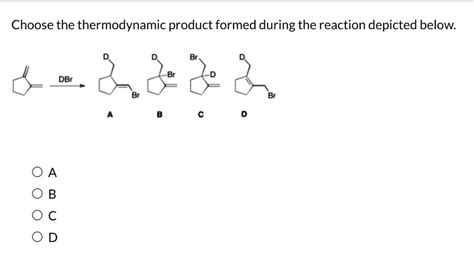
Table of Contents
Choosing the Thermodynamic Product: A Deep Dive into Reaction Thermodynamics
Understanding reaction thermodynamics is crucial in organic chemistry. Often, reactions can yield multiple products, and predicting which product will be favored—the kinetic or the thermodynamic product—requires a nuanced understanding of reaction mechanisms and energy considerations. This article delves deep into the factors influencing product formation, focusing on how to identify the thermodynamic product. We will explore various reaction types, analyze energy profiles, and provide practical strategies for predicting the dominant product.
Understanding Kinetic vs. Thermodynamic Control
Before we dive into identifying the thermodynamic product, let's clarify the distinction between kinetic and thermodynamic control. These terms describe the factors governing the product distribution in a reaction.
Kinetic Control
A kinetically controlled reaction favors the product that forms fastest. This means the reaction pathway with the lowest activation energy (Ea) is dominant. The transition state leading to the kinetic product is lower in energy than the transition state leading to the thermodynamic product. Kinetic control is often observed at low temperatures and/or short reaction times, conditions that don't allow enough time for the initially formed product to isomerize to a more stable form.
Thermodynamic Control
In contrast, a thermodynamically controlled reaction favors the product that is most stable. This is the product with the lowest Gibbs free energy (ΔG). Thermodynamic control is favored at higher temperatures and/or longer reaction times, allowing sufficient time for the initial products to equilibrate and rearrange to form the most stable isomer. The equilibrium favors the thermodynamic product.
Identifying the Thermodynamic Product: Key Factors
Several factors influence whether a reaction is under kinetic or thermodynamic control and, consequently, which product predominates.
1. Temperature: The Decisive Factor
Temperature plays a pivotal role in determining whether a reaction proceeds under kinetic or thermodynamic control. Higher temperatures generally favor thermodynamic control because they provide sufficient energy for the system to overcome the activation energy barrier and reach equilibrium, where the more stable thermodynamic product is favored. Conversely, lower temperatures often lead to kinetic control as the reaction proceeds faster along the pathway with the lower activation energy, regardless of product stability.
2. Reaction Time: Allowing for Equilibrium
Similar to temperature, reaction time significantly impacts product distribution. Longer reaction times allow for the initial products to interconvert, eventually favoring the more stable thermodynamic product. Shorter reaction times, on the other hand, might not provide enough time for equilibration, leading to a predominance of the kinetically favored product.
3. Reaction Mechanism: Unveiling the Pathways
Understanding the reaction mechanism is critical in predicting product formation. The mechanism dictates the pathway the reaction takes, influencing the activation energy barriers and the stability of intermediates and products. For instance, a reaction involving a carbocation intermediate might lead to different products depending on the stability of the carbocation. A more substituted carbocation (tertiary) is generally more stable than a less substituted carbocation (primary).
4. Gibbs Free Energy (ΔG): The Ultimate Determinant
The Gibbs free energy change (ΔG) provides a quantitative measure of the relative stability of products. A negative ΔG indicates a spontaneous reaction that favors product formation. The product with the most negative ΔG will be the thermodynamic product. This is determined by the enthalpy (ΔH) and entropy (ΔS) changes of the reaction: ΔG = ΔH - TΔS. Exothermic reactions (negative ΔH) are favored enthalpically, while reactions with a positive entropy change (increase in disorder) are favored entropically.
Examples of Thermodynamic Product Formation
Let's consider some classic examples illustrating thermodynamic product formation.
1. Alkene Addition Reactions
In electrophilic addition reactions to alkenes, the thermodynamic product is often the more substituted alkene. For instance, in the addition of HBr to an unsymmetrical alkene, the Markovnikov product (where the hydrogen atom adds to the less substituted carbon and the bromine atom adds to the more substituted carbon) is generally the major product at higher temperatures. This is because the more substituted alkene is more stable due to hyperconjugation.
2. Diels-Alder Reactions
Diels-Alder reactions, [4+2] cycloadditions, often yield a mixture of endo and exo isomers. The endo isomer is usually the kinetic product due to secondary orbital interactions during the transition state. However, under thermodynamic control (higher temperature), the exo isomer, which is sterically less hindered and therefore more stable, becomes the predominant product.
3. Claisen Rearrangement
The Claisen rearrangement is a thermally allowed [3,3]-sigmatropic rearrangement. The reaction proceeds through a concerted mechanism and often leads to the formation of a more substituted and conjugated alkene as the thermodynamic product, reflecting increased stability.
Predicting the Thermodynamic Product: A Practical Approach
To predict the thermodynamic product, consider the following steps:
- Identify all possible products: Draw all plausible structures that could form from the given reaction.
- Assess the stability of each product: Consider factors such as resonance stabilization, hyperconjugation, inductive effects, and steric hindrance. More substituted alkenes, for instance, are generally more stable.
- Consider the reaction conditions: High temperatures and long reaction times favor thermodynamic control.
- Analyze the Gibbs free energy: If possible, calculate or estimate the ΔG for each product. The product with the most negative ΔG is the thermodynamic product.
- Consult literature: Research similar reactions to see which product is generally favored under similar conditions.
Conclusion: Mastering the Art of Thermodynamic Prediction
Choosing the thermodynamic product requires a thorough understanding of reaction mechanisms, energy profiles, and the impact of reaction conditions. By carefully analyzing the stability of potential products, considering reaction parameters like temperature and time, and understanding the principles of Gibbs free energy, one can effectively predict the dominant product formed under thermodynamic control. Remember, mastering this skill is essential for effective synthesis planning and optimizing reaction outcomes. This requires not only theoretical understanding but also practical experience in performing and analyzing organic reactions. The more you practice, the more adept you will become at predicting the thermodynamic product and designing efficient synthetic strategies. Continual learning and staying abreast of the latest research in organic chemistry will further enhance your ability to accurately predict the products formed during a reaction.
Latest Posts
Latest Posts
-
Sedra Smith Microelectronic Circuits 8th Edition Solutions Pdf
Mar 21, 2025
-
Interactive Tutorial Forming Questions In Spanish
Mar 21, 2025
-
Tienes Tu Cuaderno No No 1 Of 1 Tengo
Mar 21, 2025
-
What Inference Does The Text Best Support
Mar 21, 2025
-
What Relevant Data Is Found On The Awards Eligibility Roster
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Choose The Thermodynamic Product Formed During The Reaction Depicted Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
