Classify Each Molecule As An Aldehyde Ketone Or Neither
Onlines
Mar 31, 2025 · 5 min read
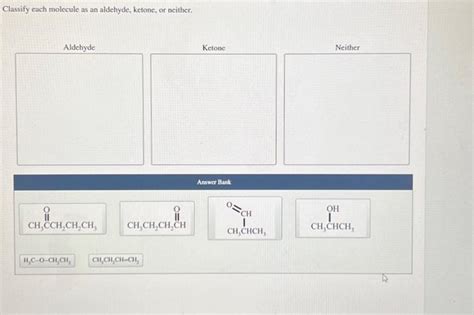
Table of Contents
Classify Each Molecule as an Aldehyde, Ketone, or Neither: A Comprehensive Guide
Identifying aldehydes and ketones is a fundamental skill in organic chemistry. These carbonyl-containing functional groups, characterized by a carbon atom double-bonded to an oxygen atom (C=O), are ubiquitous in organic molecules and exhibit distinct chemical properties. This comprehensive guide will equip you with the knowledge to confidently classify molecules as either aldehydes, ketones, or neither, explaining the underlying principles and providing numerous examples.
Understanding the Carbonyl Group: The Heart of Aldehydes and Ketones
The carbonyl group (C=O) is the defining feature of both aldehydes and ketones. The difference lies in the atoms attached to the carbonyl carbon:
-
Aldehydes: The carbonyl carbon is bonded to at least one hydrogen atom. The general formula for an aldehyde is RCHO, where R represents an alkyl or aryl group (or hydrogen in the simplest case, formaldehyde).
-
Ketones: The carbonyl carbon is bonded to two alkyl or aryl groups. The general formula for a ketone is RCOR', where R and R' represent alkyl or aryl groups. These groups can be the same or different.
Identifying Aldehydes
Identifying an aldehyde involves looking for the characteristic carbonyl group (C=O) with at least one hydrogen atom directly bonded to the carbonyl carbon.
Example 1: Formaldehyde (HCHO)
Formaldehyde is the simplest aldehyde. The carbonyl carbon is bonded to two hydrogen atoms.
Example 2: Acetaldehyde (CH₃CHO)
Acetaldehyde has a methyl group (CH₃) and a hydrogen atom bonded to the carbonyl carbon.
Example 3: Benzaldehyde (C₆H₅CHO)
Benzaldehyde features a phenyl group (C₆H₅) and a hydrogen atom bonded to the carbonyl carbon. Note that the aryl group (phenyl) counts as an 'R' group in the general formula.
Identifying Aldehydes in Complex Molecules: In larger molecules, identifying the aldehyde functional group requires careful examination of the connectivity around the carbonyl group. Look for a C=O bond where at least one of the bonds from the carbonyl carbon is to a hydrogen atom. The other bond can be to any alkyl or aryl group.
Identifying Ketones
Ketones are identified by the presence of a carbonyl group (C=O) where the carbonyl carbon is bonded to two alkyl or aryl groups. These groups can be identical (symmetrical ketones) or different (unsymmetrical ketones).
Example 1: Acetone (CH₃COCH₃)
Acetone, the simplest ketone, has two methyl groups bonded to the carbonyl carbon.
Example 2: Butanone (CH₃CH₂COCH₃)
Butanone is an example of an unsymmetrical ketone. It has an ethyl group (CH₃CH₂) and a methyl group (CH₃) bonded to the carbonyl carbon.
Example 3: Benzophenone (C₆H₅COC₆H₅)
Benzophenone is a symmetrical ketone with two phenyl groups bonded to the carbonyl carbon.
Identifying Ketones in Complex Molecules: Similar to aldehydes, identifying ketones in complex molecules requires careful attention to the bonding around the carbonyl group. If the carbonyl carbon is bonded to two carbon-containing groups (alkyl or aryl), it is a ketone.
Molecules that are Neither Aldehydes nor Ketones
Many organic molecules contain carbonyl groups but do not fall into the aldehyde or ketone classifications. These include:
-
Carboxylic Acids (RCOOH): These contain a carbonyl group bonded to a hydroxyl group (-OH).
-
Esters (RCOOR'): These have a carbonyl group bonded to an oxygen atom which is further bonded to an alkyl or aryl group.
-
Amides (RCONH₂): These possess a carbonyl group bonded to a nitrogen atom.
-
Acid Anhydrides (RCOOCOR'): These feature two carbonyl groups bonded to a single oxygen atom.
-
Acid Chlorides (RCOCl): These contain a carbonyl group bonded to a chlorine atom.
These functional groups all have carbonyl groups but differ significantly in their chemical reactivity and properties compared to aldehydes and ketones. Recognizing these distinct functional groups is crucial for predicting chemical behavior.
Practice Problems: Classifying Molecules
Let's test your understanding with some practice problems. Classify each of the following molecules as an aldehyde, ketone, or neither:
- CH₃CH₂CH₂CHO
- (CH₃)₂CHCOCH₃
- CH₃COOH
- CH₃COOCH₃
- C₆H₅CH₂CHO
- CH₃CONH₂
- (CH₃)₂CO
- CH₃CH₂CH₂CH₂COCH₃
- CH₃CH₂COCl
- HCOOH
Solutions:
- Aldehyde: The carbonyl carbon is bonded to a hydrogen and a propyl group.
- Ketone: The carbonyl carbon is bonded to an isopropyl and a methyl group.
- Neither: This is a carboxylic acid.
- Neither: This is an ester.
- Aldehyde: The carbonyl carbon is bonded to a hydrogen and a benzyl group.
- Neither: This is an amide.
- Ketone: This is acetone.
- Ketone: The carbonyl carbon is bonded to a butyl and a methyl group.
- Neither: This is an acid chloride.
- Aldehyde: This is formic acid, which is also an aldehyde.
Advanced Considerations: Recognizing Subtleties
In more complex molecules, the identification of aldehydes and ketones may require a more detailed analysis. Consider the following points:
-
Cyclic Ketones: Ketones can be part of cyclic structures (rings). Identify the carbonyl carbon and check its bonding.
-
Sterochemistry: The stereochemistry (3D arrangement of atoms) around the carbonyl group doesn't affect its classification as an aldehyde or ketone.
-
Polyfunctional Molecules: Molecules may contain multiple functional groups. Classify each functional group individually.
-
Isomerism: Isomers (molecules with the same molecular formula but different structures) can have vastly different classifications. For example, propanal (an aldehyde) and acetone (a ketone) both have the formula C₃H₆O but are very different molecules.
Conclusion
Successfully classifying molecules as aldehydes, ketones, or neither requires a solid understanding of the carbonyl group and the atoms directly attached to the carbonyl carbon. This guide provides a step-by-step approach, illustrative examples, and practice problems to solidify your understanding. Mastering this fundamental skill is crucial for advancing in organic chemistry and understanding the vast array of organic molecules and their reactions. Remember to always carefully analyze the connectivity of atoms around the carbonyl group to ensure accurate classification. By practicing diligently, you'll become proficient in identifying these important functional groups within diverse and complex organic structures.
Latest Posts
Latest Posts
-
Intersectional Chicana Feminisms Sitios Y Lenguas Pdf
Apr 01, 2025
-
Symbols In Perks Of Being A Wallflower
Apr 01, 2025
-
Summary Of Balzac And The Little Chinese Seamstress
Apr 01, 2025
-
Graduate Textbook Voucher Additional Expense Form
Apr 01, 2025
-
What Happens In Chapter 11 Of To Kill A Mockingbird
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Classify Each Molecule As An Aldehyde Ketone Or Neither . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
