Complete The Synthesis Below By Selecting Or Drawing The Reagents
Onlines
Apr 07, 2025 · 6 min read
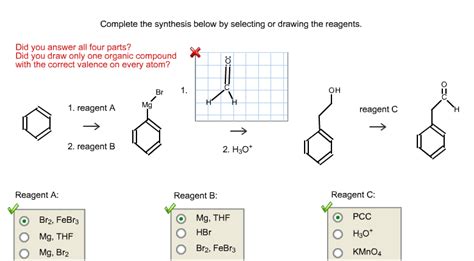
Table of Contents
Completing Organic Synthesis: A Comprehensive Guide to Reagent Selection
Organic synthesis, the art and science of constructing complex organic molecules from simpler building blocks, is a cornerstone of chemistry. Successfully navigating the complexities of synthesis hinges on a deep understanding of reaction mechanisms, functional group transformations, and, crucially, reagent selection. This article delves into the process of completing a synthesis, focusing on the strategic choices involved in selecting the appropriate reagents to achieve desired transformations. We will explore various strategies, illustrate them with examples, and provide a framework for approaching synthesis problems.
Understanding the Target Molecule and Retrosynthetic Analysis
Before even considering specific reagents, a thorough analysis of the target molecule is paramount. This involves identifying the functional groups present, their connectivity, and the overall molecular architecture. Retrosynthetic analysis, a powerful problem-solving tool, works backward from the target molecule, systematically disconnecting bonds to identify simpler precursors. This process reveals the key transformations necessary and guides the selection of suitable reagents.
For example, consider synthesizing a molecule with a ketone functionality. Retrosynthetically, we might envision disconnecting the C=O bond, leading us to consider precursors such as alcohols or aldehydes that can be oxidized to ketones.
Key Considerations in Reagent Selection
Reagent selection isn't arbitrary; it's a strategic decision based on several critical factors:
-
Functional Group Compatibility: Reagents must be compatible with all functional groups present in the starting material and any intermediate products. A reagent that might efficiently convert an alcohol to a ketone, for instance, could potentially react undesirably with an ester group in the same molecule.
-
Selectivity: Ideally, a reagent should selectively modify the desired functional group without affecting others. This is especially important in complex molecules with multiple reactive sites. Regioselectivity (preference for one position over another) and stereoselectivity (preference for one stereoisomer over another) are crucial considerations.
-
Reaction Conditions: Factors like temperature, solvent, and pH play a vital role. Some reagents require specific conditions to operate effectively, while others are more tolerant of variations.
-
Yield and Purity: High yields and the production of pure products are always desirable. Some reagents are known for their efficiency and clean reactions, minimizing the need for extensive purification.
-
Safety and Cost: Safety is paramount. Some reagents are highly toxic or flammable, requiring careful handling and specialized equipment. Cost-effectiveness is also a factor, particularly in large-scale syntheses.
Common Reagent Classes and Their Applications
Let's explore some common reagent classes and their applications in different organic transformations:
1. Oxidizing Agents:
-
PCC (Pyridinium chlorochromate): Mildly oxidizes primary alcohols to aldehydes and secondary alcohols to ketones. Known for its selectivity and relatively clean reactions.
-
Jones Reagent (CrO3/H2SO4): A stronger oxidizing agent, converting primary alcohols to carboxylic acids and secondary alcohols to ketones. Less selective than PCC.
-
Dess-Martin Periodinane (DMP): A highly selective reagent for oxidizing primary alcohols to aldehydes without over-oxidation to carboxylic acids.
-
Swern Oxidation: Utilizes DMSO, oxalyl chloride, and a base to oxidize alcohols to aldehydes or ketones. Offers good selectivity and works under mild conditions.
2. Reducing Agents:
-
Lithium Aluminum Hydride (LiAlH4): A powerful reducing agent capable of reducing a wide range of functional groups, including esters, ketones, aldehydes, and carboxylic acids to their corresponding alcohols. Reacts violently with water.
-
Sodium Borohydride (NaBH4): A milder reducing agent, primarily used for reducing aldehydes and ketones to alcohols. Compatible with protic solvents.
-
Dibal-H (Diisobutylaluminum hydride): Selectively reduces esters to aldehydes without further reduction to alcohols.
3. Grignard Reagents:
Organomagnesium halides (RMgX) are powerful nucleophiles, readily adding to carbonyl compounds (aldehydes, ketones, esters) to form new carbon-carbon bonds. They are crucial in building more complex carbon skeletons.
4. Wittig Reagents:
Phosphorous ylides (R<sub>3</sub>P=CHR) react with aldehydes and ketones to form alkenes, a versatile method for creating carbon-carbon double bonds with precise control over the stereochemistry (in some cases).
5. Protecting Groups:
Protecting groups temporarily mask reactive functional groups to prevent undesired reactions during synthesis. Common protecting groups for alcohols include:
- TBS (tert-butyldimethylsilyl): Stable under a wide range of conditions.
- Trityl (triphenylmethyl): Easily removed under acidic conditions.
- Benzyl (Bn): Removed by hydrogenation.
6. Catalysts:
Catalysts accelerate reaction rates and improve selectivity without being consumed in the reaction. Examples include:
- Palladium catalysts: Used extensively in cross-coupling reactions, such as Suzuki, Stille, and Heck couplings.
- Lewis acids: Such as AlCl3 or BF3, often used to promote electrophilic aromatic substitutions.
Strategies for Reagent Selection in Complex Syntheses
For more intricate synthetic problems, several strategies can enhance the efficiency and success of reagent selection:
-
Linear Synthesis: A straightforward approach where each step builds upon the previous one, proceeding linearly towards the target molecule.
-
Convergent Synthesis: Multiple synthetic pathways converge towards a common intermediate, which is then converted to the final product. This strategy is more efficient for complex molecules as it minimizes the cumulative impact of low yields in individual steps.
-
Protecting Group Strategies: Carefully planned use of protecting groups is essential for controlling the reactivity of multiple functional groups within a molecule. Choosing the appropriate protecting group based on compatibility with subsequent reactions is crucial.
-
One-Pot Reactions: Multiple reactions are performed sequentially in a single reaction vessel, minimizing purification steps and improving efficiency.
Illustrative Examples of Reagent Selection
Let's illustrate reagent selection with a few examples:
Example 1: Synthesis of a Ketone from an Alcohol
To synthesize a ketone from a secondary alcohol, one could use PCC or Jones reagent. PCC is preferred for its higher selectivity, preventing over-oxidation.
Example 2: Synthesis of an Alkene from an Aldehyde
The Wittig reaction offers a powerful method for synthesizing alkenes from aldehydes using a suitable Wittig reagent. The choice of reagent dictates the specific alkene formed.
Example 3: Synthesis of an Alcohol from an Ester
LiAlH4 is a strong reducing agent that efficiently reduces esters to alcohols. However, the presence of other reducible functional groups in the molecule might necessitate a milder approach or the use of protecting groups.
Conclusion: A Holistic Approach to Synthesis
Successfully completing an organic synthesis requires a holistic approach that integrates a deep understanding of reaction mechanisms, retrosynthetic analysis, and strategic reagent selection. The factors discussed here – functional group compatibility, selectivity, reaction conditions, yield, safety, and cost – are all interconnected and must be carefully considered when designing a synthetic route. Mastering this intricate dance of chemical transformations is the hallmark of a proficient synthetic chemist. Through careful planning and the appropriate application of knowledge, challenging synthetic problems can be effectively addressed and complex molecules synthesized with efficiency and precision. The ability to select the right reagents is not just a technical skill but a creative process that requires intuition and problem-solving abilities honed through practice and experience. Continued exploration of reaction mechanisms, reagent properties, and emerging synthetic techniques will further expand the chemist’s toolkit and enhance our ability to construct even more sophisticated molecules.
Latest Posts
Latest Posts
-
The Murder Of Roger Ackroyd Characters
Apr 08, 2025
-
Identify The True And False Statements About Race
Apr 08, 2025
-
What Does Fences Symbolism In Fences
Apr 08, 2025
-
Attitudes And Behaviors Come From Our Blank System
Apr 08, 2025
-
Ap Euro Unit 4 Progress Check Mcq
Apr 08, 2025
Related Post
Thank you for visiting our website which covers about Complete The Synthesis Below By Selecting Or Drawing The Reagents . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.