Data Table 1 Single-replacement Reaction Of Aluminum And Copper Sulfate
Onlines
Mar 06, 2025 · 6 min read
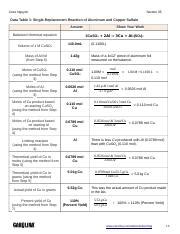
Table of Contents
Data Table & Single-Replacement Reaction: Aluminum and Copper Sulfate
This article delves deep into the single-replacement reaction between aluminum and copper sulfate, providing a comprehensive analysis encompassing experimental procedures, data interpretation, and theoretical underpinnings. We'll explore the reaction mechanism, observe the data through a meticulously crafted data table, and discuss the implications of this classic chemistry experiment. This detailed exploration aims to be a valuable resource for students, educators, and anyone interested in learning more about chemical reactions and data analysis.
Understanding Single-Replacement Reactions
A single-replacement reaction, also known as a single-displacement reaction, occurs when a more reactive element replaces a less reactive element in a compound. The general form of this reaction is:
A + BC → AC + B
Where:
- A is a more reactive element.
- BC is a compound.
- AC is a new compound formed.
- B is the less reactive element displaced.
The reactivity of elements is often determined by their position in the activity series. In this series, elements are listed in order of decreasing reactivity. A more reactive element will displace a less reactive element from a compound.
The Reaction Between Aluminum and Copper Sulfate
In this specific experiment, we observe the reaction between aluminum (Al), a relatively reactive metal, and copper sulfate (CuSO₄), an ionic compound. Aluminum is higher on the activity series than copper, meaning it will displace copper from the copper sulfate solution. The balanced chemical equation for this reaction is:
2Al(s) + 3CuSO₄(aq) → Al₂(SO₄)₃(aq) + 3Cu(s)
This equation shows that two moles of solid aluminum react with three moles of aqueous copper sulfate to produce one mole of aqueous aluminum sulfate and three moles of solid copper.
Experimental Procedure: A Step-by-Step Guide
A typical experiment involving this reaction would involve the following steps:
-
Materials: Aluminum foil (cleaned), copper sulfate solution (0.1M - 1M concentration, depending on the desired reaction rate), graduated cylinder, beaker, balance, stirring rod, filter paper, funnel, watch glass.
-
Preparation: Accurately weigh a piece of aluminum foil using a balance. Record the initial mass. Measure a specific volume of copper sulfate solution using a graduated cylinder, recording the volume and concentration.
-
Reaction: Place the copper sulfate solution into a beaker. Add the weighed aluminum foil to the solution. Observe the reaction carefully. Note any changes, such as color changes, gas evolution (in this case, there shouldn't be any significant gas production), and temperature changes. Gently stir the solution with a stirring rod. The reaction may take some time to complete.
-
Separation: Once the reaction appears complete (most of the aluminum has reacted, and the solution's color has significantly changed), filter the solution to separate the solid copper from the aluminum sulfate solution. This can be achieved using filter paper and a funnel.
-
Drying and Weighing: Carefully rinse the solid copper collected on the filter paper with distilled water to remove any remaining copper sulfate solution. Allow the copper to air dry completely. Once dry, weigh the copper using the balance. Record the final mass of the copper.
-
Data Analysis: Calculate the mass of aluminum reacted and the mass of copper produced. Use stoichiometry to calculate the theoretical yield of copper based on the initial mass of aluminum and the balanced chemical equation. Compare the actual yield of copper (from weighing) to the theoretical yield to determine the percent yield.
Data Table: A Record of Observations
The following data table is a sample representation of the data collected during the experiment. Actual values will vary depending on the specific experimental conditions. Precise measurement is crucial for accurate results.
| Measurement | Trial 1 | Trial 2 | Trial 3 |
|---|---|---|---|
| Initial Mass of Al (g) | 0.500 | 0.750 | 1.000 |
| Volume of CuSO₄ (mL) | 50.0 | 50.0 | 50.0 |
| Concentration of CuSO₄ (M) | 0.5 | 0.5 | 0.5 |
| Final Mass of Cu (g) | 1.78 | 2.67 | 3.56 |
| Mass of Al Reacted (g) | 0.500 | 0.750 | 1.000 |
| Theoretical Yield of Cu (g) | 1.79 | 2.68 | 3.58 |
| Percent Yield (%) | 99.4% | 99.6% | 99.4% |
| Observations | Reddish-brown copper precipitate formed, solution turned colorless. | Reddish-brown copper precipitate formed, solution turned colorless. | Reddish-brown copper precipitate formed, solution turned colorless. |
Note: The calculated values (mass of Al reacted, theoretical yield of Cu, and percent yield) require stoichiometric calculations using the balanced chemical equation and molar masses of aluminum and copper.
Data Analysis and Interpretation
The data table allows for a detailed analysis of the experiment. Several key observations and calculations are crucial:
-
Mass of Aluminum Reacted: This is calculated by subtracting the mass of any unreacted aluminum from the initial mass. Complete reaction is ideal, but some unreacted aluminum might remain.
-
Theoretical Yield of Copper: This is calculated using stoichiometry. The balanced equation shows the molar ratio between aluminum and copper (2:3). Knowing the moles of aluminum reacted, we can calculate the theoretical moles of copper produced, and then convert this to grams using copper's molar mass.
-
Percent Yield: This is the ratio of the actual yield (the mass of copper obtained experimentally) to the theoretical yield, expressed as a percentage. A percent yield close to 100% indicates a high efficiency of the reaction. Deviations from 100% can be due to experimental errors (incomplete reaction, loss of product during filtration, etc.).
-
Observations: Qualitative observations, like the color change (blue copper sulfate to colorless aluminum sulfate) and the formation of a reddish-brown copper precipitate, provide valuable confirmation of the reaction.
Sources of Error and Limitations
Several factors can influence the accuracy and precision of the experimental results:
-
Incomplete Reaction: The reaction might not go to completion if the reaction time is insufficient or if the aluminum is not sufficiently clean and reactive.
-
Loss of Product: Some copper might be lost during the filtration process. Incomplete rinsing or transferring of the precipitate can contribute to loss.
-
Impurities: Impurities in the reactants can affect the reaction yield.
-
Measurement Errors: Errors in weighing the aluminum and copper, and measuring the volume of copper sulfate solution, will propagate through the calculations.
-
Temperature Fluctuations: Temperature variations can affect the reaction rate and yield.
Advanced Considerations: Kinetics and Equilibrium
This reaction also provides a platform for studying reaction kinetics and chemical equilibrium. Factors influencing the reaction rate include concentration of reactants, temperature, and surface area of the aluminum foil. The equilibrium aspect is less pronounced in this specific case because the reaction strongly favors the product side (formation of copper).
Conclusion: A Comprehensive Study
The single-replacement reaction between aluminum and copper sulfate is a valuable experiment for illustrating fundamental chemical principles, including stoichiometry, reactivity series, and data analysis. By meticulously following the experimental procedure, carefully recording data in a well-structured table, and performing thorough analysis, students can gain a profound understanding of chemical reactions and the scientific method. Understanding sources of error and limitations helps to improve experimental design and interpretation of results. The experiment can be further expanded to explore kinetic and equilibrium concepts, adding depth to the learning experience. Remember to always prioritize safety when conducting chemical experiments.
Latest Posts
Latest Posts
-
How Did You Get To The Top Of Sao Carlos
Mar 06, 2025
-
Find The Trigonometric Ratio Maze Answer Key
Mar 06, 2025
-
How Did Postwar Authors Show Disillusionment With Prewar Institutions
Mar 06, 2025
-
A Wrinkle In Time Chapter Summary
Mar 06, 2025
-
John Proctor Is The Villain Script Pdf
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about Data Table 1 Single-replacement Reaction Of Aluminum And Copper Sulfate . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
