Data Table 2 Vsepr Names And Atoms
Onlines
Mar 26, 2025 · 7 min read
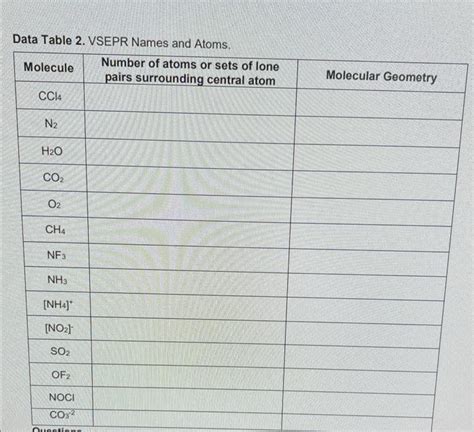
Table of Contents
Data Table: VSEPR Names, Atoms, and Molecular Geometries
Understanding molecular geometry is crucial in chemistry, impacting properties like reactivity, polarity, and physical state. The Valence Shell Electron Pair Repulsion (VSEPR) theory provides a simple yet powerful model to predict these shapes. This article delves deep into VSEPR theory, providing a comprehensive data table correlating VSEPR names, constituent atoms, and the resulting molecular geometries. We'll explore the underlying principles, exceptions, and applications of this fundamental concept.
Understanding VSEPR Theory
VSEPR theory posits that the arrangement of electron pairs (both bonding and lone pairs) around a central atom minimizes electron-electron repulsion, leading to a specific molecular geometry. The repulsive forces, in order of strength, are: lone pair-lone pair > lone pair-bonding pair > bonding pair-bonding pair. This hierarchy directly influences the resulting molecular shape.
Key Terms and Definitions:
- Central Atom: The atom around which other atoms are bonded.
- Bonding Pair: A pair of electrons shared between two atoms in a covalent bond.
- Lone Pair: A pair of electrons not involved in bonding; they reside solely on the central atom.
- Electron Domains: Regions of electron density around the central atom. This includes both bonding pairs and lone pairs.
- Molecular Geometry: The three-dimensional arrangement of atoms in a molecule. This differs from electron domain geometry if lone pairs are present.
The VSEPR Data Table: A Comprehensive Guide
The table below outlines various VSEPR notations, the number of electron domains, the number of bonding pairs and lone pairs, and the corresponding molecular geometries. Remember that the electron domain geometry describes the arrangement of all electron domains (bonding and lone pairs), while the molecular geometry describes the arrangement of only the atoms.
| VSEPR Notation | Electron Domains | Bonding Pairs | Lone Pairs | Electron Domain Geometry | Molecular Geometry | Example Molecule |
|---|---|---|---|---|---|---|
| AX₂ | 2 | 2 | 0 | Linear | Linear | BeCl₂ |
| AX₃ | 3 | 3 | 0 | Trigonal Planar | Trigonal Planar | BF₃ |
| AX₂E | 3 | 2 | 1 | Trigonal Planar | Bent/Angular | SO₂ |
| AX₄ | 4 | 4 | 0 | Tetrahedral | Tetrahedral | CH₄ |
| AX₃E | 4 | 3 | 1 | Tetrahedral | Trigonal Pyramidal | NH₃ |
| AX₂E₂ | 4 | 2 | 2 | Tetrahedral | Bent/Angular | H₂O |
| AX₅ | 5 | 5 | 0 | Trigonal Bipyramidal | Trigonal Bipyramidal | PCl₅ |
| AX₄E | 5 | 4 | 1 | Trigonal Bipyramidal | Seesaw | SF₄ |
| AX₃E₂ | 5 | 3 | 2 | Trigonal Bipyramidal | T-shaped | ClF₃ |
| AX₂E₃ | 5 | 2 | 3 | Trigonal Bipyramidal | Linear | XeF₂ |
| AX₆ | 6 | 6 | 0 | Octahedral | Octahedral | SF₆ |
| AX₅E | 6 | 5 | 1 | Octahedral | Square Pyramidal | BrF₅ |
| AX₄E₂ | 6 | 4 | 2 | Octahedral | Square Planar | XeF₄ |
Note: 'A' represents the central atom, 'X' represents the surrounding atoms, and 'E' represents lone pairs of electrons.
Detailed Explanation of Geometries and Examples:
Let's examine some key geometries in more detail:
1. Linear Geometry (AX₂, AX₂E₃):
- AX₂: Two bonding pairs arranged 180° apart. Example: BeCl₂. The beryllium atom is the central atom, with two chlorine atoms bonded to it.
- AX₂E₃: Two bonding pairs and three lone pairs. The lone pairs occupy equatorial positions in the trigonal bipyramid, forcing the bonding pairs into a linear arrangement. Example: XeF₂.
2. Trigonal Planar Geometry (AX₃, AX₂E):
- AX₃: Three bonding pairs arranged 120° apart in a plane. Example: BF₃. The boron atom is at the center, with three fluorine atoms surrounding it.
- AX₂E: Two bonding pairs and one lone pair. The lone pair occupies one of the three positions in the trigonal planar arrangement, causing the bonding pairs to compress and resulting in a bent or angular shape. Example: SO₂.
3. Tetrahedral Geometry (AX₄, AX₃E, AX₂E₂):
- AX₄: Four bonding pairs arranged tetrahedrally with 109.5° bond angles. Example: CH₄ (methane). The carbon atom is central, with four hydrogen atoms surrounding it.
- AX₃E: Three bonding pairs and one lone pair. The lone pair pushes the bonding pairs closer together, resulting in a trigonal pyramidal shape. Example: NH₃ (ammonia).
- AX₂E₂: Two bonding pairs and two lone pairs. The two lone pairs repel each other strongly, resulting in a bent or angular shape with a bond angle less than 109.5°. Example: H₂O (water).
4. Trigonal Bipyramidal Geometry (AX₅, AX₄E, AX₃E₂, AX₂E₃):
- AX₅: Five bonding pairs arranged in a trigonal bipyramid. There are two distinct positions: three equatorial positions (120° apart) and two axial positions (180° apart). Example: PCl₅.
- AX₄E: Four bonding pairs and one lone pair. The lone pair usually occupies an equatorial position to minimize repulsion, leading to a seesaw shape. Example: SF₄.
- AX₃E₂: Three bonding pairs and two lone pairs. The two lone pairs occupy equatorial positions, resulting in a T-shaped molecule. Example: ClF₃.
- AX₂E₃: Two bonding pairs and three lone pairs. The three lone pairs occupy equatorial positions, resulting in a linear molecule. Example: XeF₂.
5. Octahedral Geometry (AX₆, AX₅E, AX₄E₂):
- AX₆: Six bonding pairs arranged octahedrally, with 90° angles between adjacent bonds. Example: SF₆.
- AX₅E: Five bonding pairs and one lone pair. The lone pair distorts the octahedron, leading to a square pyramidal shape. Example: BrF₅.
- AX₄E₂: Four bonding pairs and two lone pairs. The two lone pairs are positioned opposite each other, resulting in a square planar shape. Example: XeF₄.
Exceptions to VSEPR Theory:
While VSEPR theory provides an excellent framework for predicting molecular geometries, there are exceptions. These exceptions often arise due to factors not explicitly considered in the basic VSEPR model, such as:
- d-orbital involvement: Transition metal complexes often deviate from VSEPR predictions due to the involvement of d-orbitals in bonding.
- Steric effects: Bulky substituents can cause distortions in bond angles to minimize steric hindrance.
- Multiple bonds: Double and triple bonds occupy more space than single bonds, leading to slight deviations from ideal bond angles.
Applications of VSEPR Theory:
VSEPR theory has broad applications in various fields, including:
- Predicting molecular polarity: The geometry of a molecule, determined by VSEPR, significantly influences its polarity. Symmetrical molecules are often nonpolar, while asymmetrical molecules are usually polar.
- Understanding chemical reactivity: Molecular geometry plays a vital role in determining the reactivity of a molecule. The accessibility of atoms and the presence of lone pairs can significantly influence reaction pathways.
- Spectroscopy: VSEPR predictions aid in interpreting spectroscopic data, such as infrared (IR) and Raman spectroscopy, by providing insights into the vibrational modes of molecules.
- Materials science: Understanding molecular geometry is essential in designing materials with specific properties. For instance, VSEPR can help predict the packing of molecules in crystals, which impacts material strength and other properties.
Conclusion:
VSEPR theory provides a fundamental and widely used tool for predicting molecular geometries. By considering the number of electron domains, both bonding and lone pairs, around a central atom, we can accurately estimate the three-dimensional arrangement of atoms within a molecule. This knowledge is critical for understanding a wide range of chemical and physical properties. Although exceptions exist, the VSEPR model remains an invaluable tool in chemistry, providing a simple yet powerful means to understand the structure and behavior of molecules. This comprehensive data table and explanation aim to solidify your understanding of this crucial concept. Remember that consistent practice and application are key to mastering VSEPR theory and its predictions.
Latest Posts
Latest Posts
-
Beat The Clock Time Management Training Article
Mar 29, 2025
-
Summary Of Chapter 7 The Giver
Mar 29, 2025
-
Which Text Evidence Best Supports The Authors Claim And Purpose
Mar 29, 2025
-
Counselors Practice Unintentional Racism When They
Mar 29, 2025
-
Reinforcement For Emitting A Correct Echoic Is Usually
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about Data Table 2 Vsepr Names And Atoms . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
