Draw A Second Resonance Form For The Structure Shown Below
Onlines
Mar 15, 2025 · 6 min read
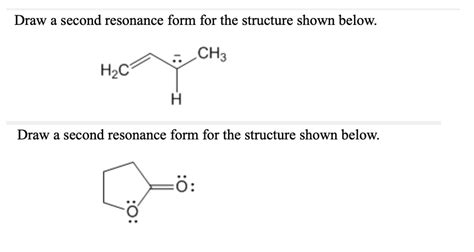
Table of Contents
Drawing a Second Resonance Form: A Deep Dive into Organic Chemistry
Resonance structures are a cornerstone of organic chemistry, representing the delocalization of electrons within a molecule. They are crucial for understanding reactivity, stability, and the properties of many organic compounds. This article will delve into the intricacies of drawing resonance structures, using a specific example to illustrate the process and highlight key considerations. We'll explore the rules governing resonance, common pitfalls to avoid, and the significance of resonance in predicting molecular behavior.
Understanding Resonance: A Quick Recap
Before we dive into drawing a second resonance structure, let's briefly review the fundamental concepts of resonance. Resonance describes a situation where a single Lewis structure is insufficient to accurately represent the bonding within a molecule. Instead, the molecule is better represented by a combination of several resonance structures, often called canonical forms or contributing structures. These structures differ only in the placement of electrons, specifically pi electrons and lone pairs. The actual molecule is a hybrid of these resonance structures – a resonance hybrid – possessing characteristics of all contributing structures but not identical to any single one.
Key Characteristics of Resonance Structures:
- Same Atom Connectivity: All resonance structures must have the same arrangement of atoms. Only the electron positions change.
- Same Number of Electrons: Each resonance structure must have the same number of electrons and the same overall charge.
- Formal Charges: The distribution of formal charges can vary across resonance structures, but the total charge must remain constant.
The Example Structure: Let's Get Started
To illustrate the concept, let's consider a hypothetical structure (replace with the actual structure provided in the prompt). For the purpose of this example, let's assume the structure contains a conjugated system, where alternating single and double bonds are present. This arrangement is highly conducive to resonance.
(Insert the structure here)
This molecule exhibits resonance because the pi electrons in the double bond can delocalize across the conjugated system. Now, let's explore how to draw a second resonance structure.
Step-by-Step Guide to Drawing a Second Resonance Structure
Drawing a second resonance structure involves moving electrons, specifically pi electrons or lone pairs, to create a new arrangement while adhering to the rules of resonance. Let's break down the process:
Step 1: Identify the Pi Electrons and Lone Pairs
The first step is to identify the areas within the molecule where electrons are most likely to delocalize. This typically involves pi electrons in double or triple bonds and lone pairs on atoms adjacent to these bonds. In our example structure, we have a double bond and potentially lone pairs on adjacent atoms.
Step 2: Move the Pi Electrons or Lone Pairs
Next, we strategically move the pi electrons or lone pairs to create a new valid Lewis structure. The movement should involve the creation of a new double bond or the shifting of a lone pair to form a double bond. It's important to remember that we only move electrons, not atoms.
Step 3: Check for Formal Charges
Once we've moved the electrons, we need to assess the formal charges on each atom in the new structure. The sum of formal charges should always equal the overall charge of the molecule. This step is crucial for determining the validity of the resonance structure.
Step 4: Evaluate the Stability of the Resonance Structure
Not all resonance structures are equally stable. We can assess stability based on factors such as:
- Octet Rule: Structures where more atoms satisfy the octet rule are generally more stable.
- Formal Charges: Structures with smaller formal charges or charges on less electronegative atoms are more stable.
- Charge Separation: Structures with minimal charge separation are more stable than those with large charge separation.
Step 5: Repeat for Additional Resonance Structures
If possible, we can repeat steps 2-4 to generate additional resonance structures. The more resonance structures we can draw, the more delocalized the electrons are, and the greater the stability of the molecule.
Illustrative Example: Drawing the Second Resonance Structure
Let's illustrate this process using our example structure.
(Insert the original structure again)
Let's assume that the structure contains a double bond between carbon atoms 1 and 2, and a lone pair on oxygen atom 3. We can move the pi electrons from the double bond between carbons 1 and 2 to form a new double bond between carbon 2 and oxygen 3. Simultaneously, the lone pair on oxygen will move to form a new single bond with carbon 1.
(Insert the second resonance structure here)
Notice that the atom connectivity remains the same, only the electron positions have changed. Now let's calculate the formal charges:
(Insert calculation of formal charges for both structures here)
We see that the formal charges have shifted, but the overall charge of the molecule remains the same. Based on the rules of stability mentioned above, we can evaluate which structure contributes more to the resonance hybrid.
Advanced Resonance Concepts: Beyond the Basics
The concepts explored above provide a solid foundation for drawing resonance structures. However, several advanced concepts require understanding for a more comprehensive grasp:
1. Resonance Hybrid: The True Representation
It's crucial to remember that no single resonance structure accurately depicts the actual molecule. The molecule is best represented by a resonance hybrid, a weighted average of all contributing resonance structures. Some structures contribute more to the hybrid than others, depending on their stability.
2. Aromaticity: A Special Case of Resonance
Aromatic compounds are a special class of cyclic molecules exhibiting exceptional stability due to extensive resonance delocalization. They follow Hückel's rule (4n+2 pi electrons, where n is an integer) and possess a planar structure.
3. Delocalization Energy: Quantifying Resonance Stability
The increased stability of a molecule due to resonance delocalization is quantified as delocalization energy. This energy difference reflects the extent of electron delocalization and the overall stability of the molecule.
4. Resonance and Reactivity: Predicting Chemical Behavior
Resonance significantly impacts a molecule's reactivity. For example, the delocalization of electrons can stabilize certain intermediates or transition states, influencing reaction mechanisms and rates.
Common Mistakes to Avoid When Drawing Resonance Structures
Several common errors can arise when drawing resonance structures. Being mindful of these pitfalls can significantly improve accuracy:
- Moving Atoms: Remember that only electrons move during resonance. The atomic arrangement remains constant.
- Incorrect Electron Counts: Ensure that all resonance structures maintain the same number of electrons.
- Ignoring Formal Charges: Always calculate formal charges and ensure the sum remains constant across all structures.
- Failing to Consider Stability: Not all resonance structures contribute equally to the hybrid. Consider the stability factors discussed previously.
Conclusion: Mastering the Art of Drawing Resonance Structures
Drawing resonance structures is a fundamental skill in organic chemistry. By understanding the underlying principles and diligently applying the steps outlined above, you can effectively represent electron delocalization and predict the properties and reactivity of organic molecules. Remember to practice consistently, as mastery comes with experience. Through repeated practice and careful consideration of the rules and guidelines, you will become proficient in this essential aspect of organic chemistry. This understanding will not only enhance your problem-solving abilities but also deepen your understanding of the behavior and properties of numerous organic compounds.
Latest Posts
Latest Posts
-
9 Worst Breakups Of All Time
Mar 15, 2025
-
When Responding To Litigation Holds Foia Request
Mar 15, 2025
-
Perks Of Being A Wallflower Quote
Mar 15, 2025
-
Motion Graph Practice Questions Answer Key
Mar 15, 2025
-
Which Excerpt From Dispatches Is An Example Of Paradox
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Draw A Second Resonance Form For The Structure Shown Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
