Draw The Correct Product For The Given Diels-alder Reaction
Onlines
Mar 29, 2025 · 5 min read
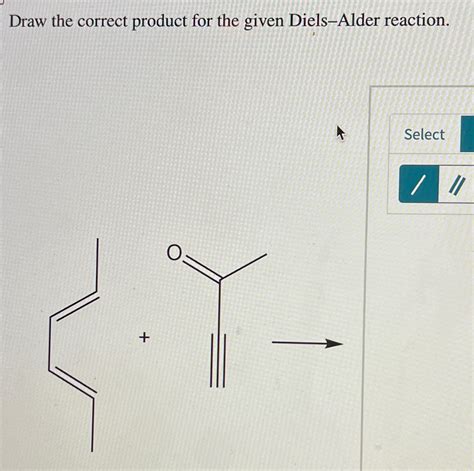
Table of Contents
Drawing the Correct Product for a Diels-Alder Reaction: A Comprehensive Guide
The Diels-Alder reaction, a cornerstone of organic chemistry, is a powerful tool for forming six-membered rings. Understanding how to predict the product of this [4+2] cycloaddition is crucial for any organic chemist. This comprehensive guide will walk you through the process, covering stereochemistry, regiochemistry, and common pitfalls. We'll explore various examples and provide you with the tools to confidently draw the correct product for any given Diels-Alder reaction.
Understanding the Basics: Dienes and Dienophiles
Before diving into predicting products, let's review the essential components:
The Diene: The 4π Electron System
The diene is a conjugated system containing four π electrons. Crucially, it must be able to adopt an s-cis conformation, meaning the two double bonds are on the same side of a single bond. This conformation is necessary for the concerted cycloaddition. Examples include:
- 1,3-Butadiene: The simplest and most common diene.
- Cyclopentadiene: A highly reactive cyclic diene. Its reactivity is enhanced by the relief of ring strain upon cycloaddition.
- 1-methoxy-1,3-butadiene: Electron-rich dienes react faster.
- Anthracene: A polycyclic aromatic hydrocarbon that can act as a diene in certain circumstances.
The Dienophile: The 2π Electron System
The dienophile is a molecule containing a double or triple bond (a 2π electron system). Electron-withdrawing groups on the dienophile increase its reactivity. Examples include:
- Ethene: The simplest dienophile.
- Acrylonitrile: Contains an electron-withdrawing cyano group, making it a highly reactive dienophile.
- Maleic anhydride: A cyclic dienophile with two electron-withdrawing carbonyl groups.
- N-Phenylmaleimide: A highly reactive dienophile due to the electron-withdrawing effects of the phenyl and imide groups.
Predicting the Product: Stereochemistry and Regiochemistry
The Diels-Alder reaction is a concerted process, meaning all bond breaking and bond forming occurs in a single step. This concerted nature dictates the stereochemistry and regiochemistry of the product.
Stereochemistry: A Stereoselective Reaction
The Diels-Alder reaction is stereospecific and stereoselective. This means that the stereochemistry of the reactants directly influences the stereochemistry of the product.
-
endo vs exo: When a dienophile has substituents, the product can exist as two isomers: endo and exo. The endo isomer has the substituents on the dienophile oriented towards the newly formed bridgehead carbons. The exo isomer has the substituents oriented away from the bridgehead carbons. The endo product is generally favored due to secondary orbital interactions (though this isn't always the case). Understanding this concept is key to drawing the correct product.
-
cis/trans relationship: The cis/trans relationship of substituents on the diene and dienophile are preserved in the product. A cis substituent on the diene will remain cis in the product, and a trans substituent will remain trans.
Regiochemistry: Predicting the Position of Substituents
Regiochemistry refers to the relative positions of substituents in the product. The most stable product will be formed, following the principle of maximizing the electron-donating effects on the diene and electron-withdrawing effects on the dienophile. This can be predicted using simple guidelines:
- Electron-rich dienes and electron-poor dienophiles: The electron-rich diene's substituents will preferentially bond to the carbon atom of the dienophile bearing the electron-withdrawing group. This increases the stability of the transition state.
- Electron-poor dienes and electron-rich dienophiles: The electron-poor diene's substituents will preferentially bond to the carbon atom of the dienophile bearing the electron-donating group. Again, this optimizes stability.
Working Through Examples: Step-by-Step Predictions
Let's work through some examples to solidify our understanding:
Example 1: A Simple Reaction
Reactants: 1,3-butadiene + ethene
Product: Cyclohexene
This is a straightforward example. No regiochemical issues arise since both reactants are unsubstituted. The product is simply cyclohexene.
Example 2: Introducing Regiochemistry
Reactants: 1-methoxy-1,3-butadiene + acrylonitrile
Product: A substituted cyclohexene. The methoxy group on the diene will be adjacent to the cyano group on the dienophile. This is because the electron-donating methoxy group preferentially bonds to the carbon atom bearing the electron-withdrawing cyano group.
Example 3: Incorporating Stereochemistry
Reactants: Cyclopentadiene + maleic anhydride
Product: Two possible stereoisomers, the endo isomer will be the major product. Both the double bonds in the reactants have a cis relationship, meaning the product's substituents will be cis to each other. The endo isomer is favoured due to secondary orbital overlap between the carbonyl groups on the maleic anhydride and the diene.
Example 4: A More Complex Scenario
Reactants: 2-methyl-1,3-butadiene + ethyl acrylate
Product: Several possible regio- and stereoisomers are possible here. Carefully consider both the regiochemistry (the methyl group is adjacent to the ester group) and stereochemistry. The endo stereoisomer is likely favoured. The methyl and ethoxycarbonyl group will be cis to each other.
Troubleshooting Common Mistakes
- Forgetting the s-cis conformation: Ensure the diene is in the correct conformation before attempting the reaction.
- Ignoring stereochemistry: Always account for the cis/trans relationship of substituents.
- Misinterpreting endo/exo selectivity: Remember that endo selectivity is generally favored but not always guaranteed. Consider the specific reactants involved.
- Neglecting regiochemistry: Carefully evaluate the electron-donating/withdrawing effects of substituents on both the diene and the dienophile.
Advanced Considerations: Beyond the Basics
- Inverse Electron Demand Diels-Alder Reactions: In these reactions, an electron-rich dienophile reacts with an electron-poor diene. The regioselectivity will be reversed compared to the normal electron-demand reaction.
- Solvent Effects: The solvent can influence the reaction rate and selectivity. Polar solvents generally increase the rate.
- Catalyst effects: Lewis acids can catalyze Diels-Alder reactions, often enhancing both the rate and selectivity.
Conclusion: Mastering the Diels-Alder Reaction
Predicting the products of Diels-Alder reactions requires a thorough understanding of stereochemistry, regiochemistry, and the electronic properties of the reactants. By carefully considering the s-cis conformation of the diene, the electron-donating/withdrawing effects of substituents, and the endo/exo selectivity, you can confidently draw the correct product for a wide range of Diels-Alder reactions. Remember to practice, and you'll master this important reaction. Through consistent practice and a methodical approach, you'll become proficient at predicting the outcomes of these powerful reactions. This will be invaluable in your studies and future research involving organic synthesis. Remember to always meticulously analyze each reactant, paying close attention to substituent effects and stereochemical considerations to achieve accurate predictions.
Latest Posts
Latest Posts
-
Weekly Schedules For The Providers Should Be Accessible To
Mar 31, 2025
-
A Long Walk To Water Characters
Mar 31, 2025
-
Which Of The Following Is An Example Of Green Computing
Mar 31, 2025
-
Summary For Chapter 11 To Kill A Mockingbird
Mar 31, 2025
-
Which Drive Is Displayed First In The Command Window
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Draw The Correct Product For The Given Diels-alder Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
