Draw The Major Organic Product Generated In The Reaction Below
Onlines
Mar 25, 2025 · 7 min read
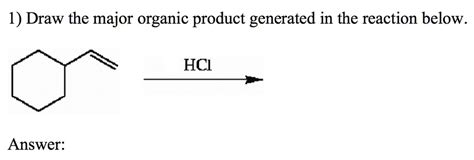
Table of Contents
Drawing the Major Organic Product: A Comprehensive Guide to Reaction Mechanisms and Product Prediction
Predicting the major organic product of a reaction is a fundamental skill in organic chemistry. This process involves understanding reaction mechanisms, recognizing functional groups, and applying established principles of reactivity and selectivity. This article delves deep into the process, providing a step-by-step approach to accurately predict the major product formed in various organic reactions. We’ll explore several common reaction types, focusing on identifying the key factors that influence product formation and applying this knowledge to complex scenarios.
Understanding Reaction Mechanisms: The Foundation of Product Prediction
Before diving into specific reactions, it's crucial to grasp the concept of reaction mechanisms. A reaction mechanism is a detailed step-by-step description of how a reaction occurs, including the movement of electrons and the formation and breaking of bonds. Understanding the mechanism allows us to predict the structure of the intermediate species and, ultimately, the final product.
Key Concepts in Reaction Mechanisms:
-
Nucleophiles and Electrophiles: Nucleophiles are electron-rich species that donate electrons to electrophiles, which are electron-deficient species. Understanding the interplay between nucleophiles and electrophiles is critical in predicting the direction of bond formation and breaking.
-
Carbocation Stability: Carbocations, positively charged carbon atoms, are common intermediates in many reactions. Their stability is determined by the number of alkyl groups attached to the positively charged carbon. Tertiary carbocations are the most stable, followed by secondary, then primary, and finally methyl carbocations. The stability of a carbocation significantly influences the regioselectivity of the reaction.
-
Stereochemistry: Stereochemistry plays a crucial role in determining the three-dimensional structure of the product. Understanding concepts like chirality, enantiomers, diastereomers, and stereospecific reactions is essential for accurate product prediction.
-
Leaving Groups: Leaving groups are atoms or groups of atoms that depart from a molecule during a reaction, taking a pair of electrons with them. Good leaving groups are generally weak bases, such as halides (Cl⁻, Br⁻, I⁻), tosylates (OTs), and mesylates (OMs). The nature of the leaving group affects the rate and outcome of the reaction.
Predicting Products in Common Reaction Types
Let's now examine some common organic reactions and apply the principles discussed above to predict their major products. Remember that without a specific reaction provided, this section provides examples. To accurately predict the product for your reaction, please provide the starting materials and reagents involved.
1. SN1 and SN2 Reactions: Nucleophilic Substitution
Nucleophilic substitution reactions involve the replacement of a leaving group by a nucleophile. Two main mechanisms are possible: SN1 (Substitution Nucleophilic Unimolecular) and SN2 (Substitution Nucleophilic Bimolecular).
-
SN1 Reactions: These reactions proceed in two steps: the leaving group departs first, forming a carbocation intermediate, followed by the nucleophile attacking the carbocation. SN1 reactions favor tertiary substrates due to the stability of the resulting tertiary carbocation. They are also favored by polar protic solvents. Racemization often occurs due to the planar nature of the carbocation intermediate.
-
SN2 Reactions: These reactions proceed in a single concerted step where the nucleophile attacks the substrate from the backside, simultaneously displacing the leaving group. SN2 reactions favor primary substrates and are favored by polar aprotic solvents. SN2 reactions result in inversion of configuration at the chiral center.
2. E1 and E2 Reactions: Elimination Reactions
Elimination reactions involve the removal of a leaving group and a proton from adjacent carbon atoms, resulting in the formation of a double bond (alkene). Like substitution reactions, two main mechanisms exist: E1 and E2.
-
E1 Reactions: These reactions proceed in two steps: the leaving group departs first, forming a carbocation intermediate, followed by the abstraction of a proton from a beta-carbon by a base. Similar to SN1 reactions, E1 reactions favor tertiary substrates and polar protic solvents. The major product is often the most substituted alkene (Zaitsev's rule).
-
E2 Reactions: These reactions proceed in a single concerted step where the base abstracts a proton from a beta-carbon, while simultaneously the leaving group departs. E2 reactions favor strong bases and can occur with primary, secondary, and tertiary substrates. The major product is often the most substituted alkene (Zaitsev's rule), although steric hindrance can influence the product distribution.
3. Addition Reactions: Electrophilic and Nucleophilic Addition
Addition reactions involve the addition of two or more atoms or groups to a molecule containing a multiple bond (alkene or alkyne).
-
Electrophilic Addition: In electrophilic addition to alkenes, an electrophile attacks the double bond, forming a carbocation intermediate. This carbocation is then attacked by a nucleophile. Markovnikov's rule dictates the regioselectivity of this reaction: the electrophile adds to the carbon atom with the greater number of hydrogen atoms.
-
Nucleophilic Addition: Nucleophilic addition to carbonyl compounds (aldehydes and ketones) involves the attack of a nucleophile on the carbonyl carbon, followed by protonation. The stereochemistry of the product is often influenced by the approach of the nucleophile.
4. Oxidation and Reduction Reactions
Oxidation and reduction reactions involve the transfer of electrons. Oxidation is the loss of electrons, while reduction is the gain of electrons. Many oxidizing and reducing agents are used in organic chemistry, and their specific reactivity determines the outcome of the reaction.
-
Oxidation of Alcohols: Primary alcohols can be oxidized to aldehydes or carboxylic acids, depending on the oxidizing agent used. Secondary alcohols are oxidized to ketones. Tertiary alcohols are resistant to oxidation.
-
Reduction of Carbonyl Compounds: Aldehydes and ketones can be reduced to primary and secondary alcohols, respectively, using reducing agents such as lithium aluminum hydride (LiAlH₄) or sodium borohydride (NaBH₄).
Applying Principles to Predict the Major Product
To accurately predict the major organic product, follow these steps:
-
Identify the Functional Groups: Determine the functional groups present in the starting material and reagents. This will help you identify the likely reaction type.
-
Identify the Reaction Type: Based on the functional groups and reagents, determine the type of reaction that will occur (SN1, SN2, E1, E2, addition, oxidation, reduction, etc.).
-
Draw the Mechanism: Draw a detailed mechanism showing the movement of electrons and the formation and breaking of bonds. This step is crucial for understanding the stereochemistry and regiochemistry of the product.
-
Consider Steric Hindrance: Steric hindrance can affect the reactivity of substrates and the selectivity of the reaction. Larger groups can hinder the approach of reactants, leading to the formation of a different product.
-
Consider the Stability of Intermediates: The stability of carbocations, carbanions, and other intermediates significantly influences the outcome of the reaction. More stable intermediates are formed preferentially.
-
Predict the Major Product: Based on the mechanism and other factors, predict the structure of the major organic product.
Advanced Considerations: Beyond the Basics
While the above provides a solid foundation, predicting products in complex reactions requires advanced knowledge and understanding of:
-
Kinetic vs. Thermodynamic Control: Some reactions can proceed through different pathways, leading to different products depending on the reaction conditions (temperature, time, etc.).
-
Protecting Groups: Protecting groups are used to temporarily mask reactive functional groups during a multi-step synthesis. Understanding the use of protecting groups is crucial for predicting the outcome of complex synthetic sequences.
-
Transition Metal Catalysis: Transition metal catalysts are used in a wide variety of organic reactions to facilitate bond formation and breaking. Understanding the catalytic cycle and the role of the metal center is crucial for predicting the product of these reactions.
-
Computational Chemistry: Computational chemistry methods can be used to predict the outcome of complex organic reactions. These methods are becoming increasingly powerful and accurate, and are often used in conjunction with experimental studies.
Conclusion: Mastering Product Prediction
Predicting the major organic product of a reaction is a challenging but rewarding aspect of organic chemistry. By carefully considering the reaction mechanism, the nature of the reactants and reagents, and other factors discussed in this article, you can significantly improve your ability to accurately predict the structure and stereochemistry of the resulting organic product. Remember that practice is key! Work through numerous examples, and don't hesitate to seek clarification when needed. With dedication and a systematic approach, mastering this skill will undoubtedly enhance your understanding and proficiency in organic chemistry. Remember to always consult your textbook and lecture notes for specific reaction details and examples. This article provides a general framework; the specific details of each reaction will depend on the exact conditions employed.
Latest Posts
Latest Posts
-
Monster By Walter Dean Myers Quotes
Mar 26, 2025
-
Ati Iv Therapy And Peripheral Access
Mar 26, 2025
-
Simone De Beauvoir The Second Sex Summary
Mar 26, 2025
-
To Kill A Mockingbird Chapter 30 Summary
Mar 26, 2025
-
5 2 Warm Up Learning Resource Knowledge Answer Key
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Organic Product Generated In The Reaction Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
