Draw The Major Product Of This Reaction.
Onlines
Mar 15, 2025 · 6 min read
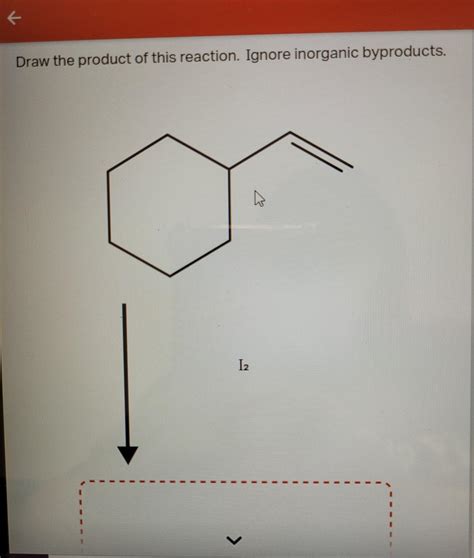
Table of Contents
Drawing the Major Product: A Deep Dive into Organic Reaction Mechanisms
Predicting the major product of a chemical reaction is a cornerstone of organic chemistry. It requires a thorough understanding of reaction mechanisms, steric hindrance, and the inherent reactivity of different functional groups. This article will delve into the process, providing a framework for accurately predicting major products in various organic reactions. We'll explore several reaction types, highlighting key concepts and considerations for accurately determining the dominant product formed. This will involve not only understanding the what of the reaction but also the why, providing a robust foundation for your organic chemistry studies.
Understanding Reaction Mechanisms: The Key to Predicting Products
Before diving into specific reactions, it's crucial to understand the underlying mechanisms. A reaction mechanism details the step-by-step process of bond breaking and bond formation. This step-wise approach is crucial because the major product is determined by the most favorable pathway – the one with the lowest activation energy. Different reaction mechanisms lead to different products. Common mechanisms include:
1. SN1 (Substitution Nucleophilic Unimolecular) Reactions:
SN1 reactions involve a two-step mechanism. The first step is the rate-determining step, where the leaving group departs, forming a carbocation intermediate. The second step involves the nucleophile attacking the carbocation. Key characteristics:
- Carbocation formation: This step is crucial. More stable carbocations (tertiary > secondary > primary) are formed more readily. The stability of the carbocation dictates the regioselectivity (which carbon atom the nucleophile attacks).
- Racemization: The planar carbocation can be attacked from either side, leading to a racemic mixture of products (unless chiral centers are already present).
- Rearrangements: Carbocation rearrangements (hydride or alkyl shifts) can occur to form more stable carbocations, potentially altering the final product.
2. SN2 (Substitution Nucleophilic Bimolecular) Reactions:
SN2 reactions occur in a single concerted step. The nucleophile attacks the carbon atom bearing the leaving group from the backside, simultaneously displacing the leaving group. Key characteristics:
- Backside attack: This leads to inversion of configuration at the stereocenter.
- Steric hindrance: Bulky groups around the reaction center hinder the backside attack, slowing down the reaction rate. Steric hindrance plays a significant role in determining the major product, as less hindered substrates react faster.
- Strong nucleophiles: SN2 reactions require strong nucleophiles.
3. E1 (Elimination Unimolecular) Reactions:
E1 reactions are similar to SN1 reactions in that they involve a carbocation intermediate. However, instead of nucleophilic attack, a base abstracts a proton, leading to the formation of a double bond (alkene). Key characteristics:
- Carbocation formation: As with SN1, the stability of the carbocation influences the product. More stable carbocations lead to more substituted alkenes (Zaitsev's rule).
- Zaitsev's rule: Predicts that the major alkene product will be the most substituted alkene (the one with the most alkyl groups attached to the double bond).
- Competition with SN1: E1 and SN1 reactions often compete, and the conditions (e.g., temperature, solvent, base concentration) can influence which pathway is favored.
4. E2 (Elimination Bimolecular) Reactions:
E2 reactions are concerted, like SN2 reactions. A base abstracts a proton while the leaving group departs simultaneously, forming a double bond. Key characteristics:
- Concerted mechanism: The reaction occurs in a single step.
- Stereochemistry: E2 reactions often exhibit stereospecificity. The proton and leaving group must be anti-periplanar (on opposite sides of the molecule) for the reaction to proceed efficiently.
- Zaitsev's rule: Generally follows Zaitsev's rule, favoring the most substituted alkene. However, steric factors can sometimes override this rule. The orientation of the base can also play a role in determining the regioselectivity of elimination. A bulky base may favor the less substituted product (Hofmann product).
Predicting the Major Product: A Step-by-Step Approach
To accurately predict the major product, follow these steps:
- Identify the functional groups: Determine the reactive functional groups present in the reactants.
- Identify the reaction type: Based on the reactants and reagents, determine the likely reaction type (SN1, SN2, E1, E2, addition, etc.).
- Draw the mechanism: Draw out the detailed mechanism, showing all intermediate steps and transition states. This is crucial for understanding the factors that determine the major product. Pay close attention to carbocation stability, steric hindrance, and stereochemistry.
- Consider competing reactions: Determine if competing reactions are possible. This is particularly important in reactions where SN1/E1 or SN2/E2 pathways are possible.
- Determine the major product: Based on your understanding of the mechanism and the relative rates of competing pathways, identify the major product. Consider factors like carbocation stability, steric effects, and the strength of the nucleophile or base.
Examples: Predicting Major Products in Specific Reactions
Let's illustrate this process with some examples:
Example 1: SN1 Reaction of 2-bromo-2-methylpropane with methanol
The tertiary carbocation formed is relatively stable, favoring an SN1 mechanism. Methanol acts as the nucleophile. The product will be a racemic mixture of 2-methoxy-2-methylpropane due to attack from either side of the planar carbocation.
Example 2: SN2 Reaction of 1-bromobutane with sodium ethoxide in ethanol
The primary alkyl halide favors an SN2 mechanism. Ethoxide acts as a strong nucleophile. The product is 1-ethoxybutane with inversion of configuration.
Example 3: E2 Reaction of 2-bromobutane with potassium tert-butoxide
Potassium tert-butoxide is a bulky base, favoring Hofmann elimination. The less substituted alkene (1-butene) will be the major product, despite Zaitsev's rule usually predicting the more substituted alkene.
Example 4: Acid-catalyzed dehydration of 2-methyl-2-propanol
The acid protonates the hydroxyl group, leading to the formation of a tertiary carbocation. Water acts as the leaving group. The major product will be 2-methylpropene, following Zaitsev's rule.
Advanced Considerations: Beyond the Basics
Several advanced concepts can further refine your ability to predict major products:
- Thermodynamic vs. Kinetic Control: Some reactions can lead to different products depending on the reaction conditions (temperature, reaction time). Thermodynamic control favors the most stable product, while kinetic control favors the product formed fastest.
- Solvent Effects: The solvent can significantly influence reaction rates and selectivities. Polar protic solvents often favor SN1 and E1 reactions, while polar aprotic solvents favor SN2 reactions.
- Protecting Groups: In complex molecules, protecting groups might be necessary to selectively react with a specific functional group while leaving others untouched. This greatly impacts the prediction of the major product.
Conclusion: Mastering the Art of Product Prediction
Predicting the major product of an organic reaction is a challenging but rewarding skill. By thoroughly understanding reaction mechanisms, considering steric effects, and recognizing potential competing pathways, you can significantly improve your accuracy. Remember to carefully analyze each reaction, step-by-step, considering all relevant factors. Through practice and a deep understanding of the underlying principles, you can master the art of predicting the major product and become a more proficient organic chemist. Consistent practice with diverse examples and a focus on understanding the why behind each reaction will solidify your skills and allow you to tackle increasingly complex problems with confidence.
Latest Posts
Latest Posts
-
Pa Kite Mwen O Bon Sove Lyrics
Mar 15, 2025
-
Chiles Major Exports Have Been
Mar 15, 2025
-
In Which Culture Is A Person Who Sees Him Herself
Mar 15, 2025
-
Summary Of A Feast For Crows
Mar 15, 2025
-
Jaime Decidir Comprar Tableta
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Draw The Major Product Of This Reaction. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
