Draw The Product Of This Hydrogenation Reaction. Ignore Inorganic Byproducts
Onlines
Mar 24, 2025 · 5 min read
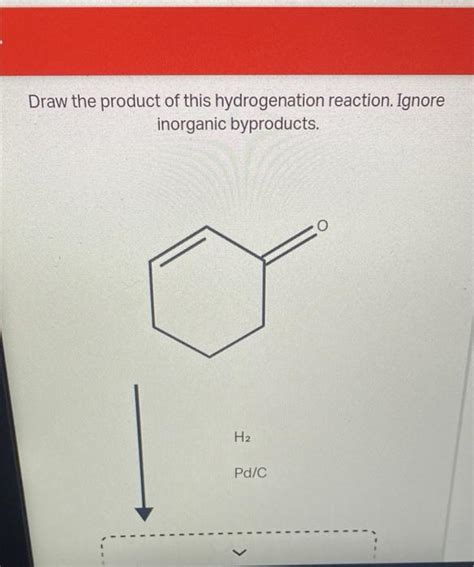
Table of Contents
Drawing the Product of Hydrogenation Reactions: A Comprehensive Guide
Hydrogenation, the process of adding hydrogen (H₂) to a molecule, is a fundamental reaction in organic chemistry with widespread applications in various industries, from the production of margarine to the synthesis of pharmaceuticals. Understanding how to predict the product of a hydrogenation reaction is crucial for any organic chemist. This comprehensive guide will delve into the intricacies of hydrogenation, focusing on drawing the products and considering various factors that influence the reaction outcome.
Understanding the Basics of Hydrogenation
Hydrogenation typically involves the addition of H₂ across a multiple bond, such as a carbon-carbon double bond (C=C) or a carbon-carbon triple bond (C≡C). This process requires a catalyst, usually a transition metal like platinum (Pt), palladium (Pd), nickel (Ni), or rhodium (Rh). These catalysts facilitate the cleavage of the strong H-H bond and the subsequent addition of hydrogen atoms to the unsaturated molecule.
The reaction is generally exothermic, meaning it releases heat. The driving force behind hydrogenation is the formation of stronger sigma (σ) bonds in the product compared to the weaker pi (π) bonds in the reactant.
Types of Hydrogenation Reactions
Several variations exist within hydrogenation reactions, depending on the substrate and reaction conditions. These include:
-
Alkene Hydrogenation: This is the most common type, involving the addition of hydrogen across a carbon-carbon double bond to form an alkane. The reaction proceeds via syn addition, meaning both hydrogen atoms add to the same side of the double bond.
-
Alkyne Hydrogenation: Alkynes, containing a carbon-carbon triple bond, can undergo hydrogenation in a stepwise manner. The first hydrogenation step typically yields a cis-alkene, which can then be further hydrogenated to an alkane. The selectivity can be controlled by the catalyst and reaction conditions.
-
Hydrogenation of Aromatic Compounds: Aromatic compounds, characterized by their delocalized pi electron system, are more resistant to hydrogenation than alkenes and alkynes. However, under more vigorous conditions and with specific catalysts, they can be hydrogenated to form cycloalkanes.
-
Asymmetric Hydrogenation: This specialized type of hydrogenation uses chiral catalysts to produce enantiomerically enriched products. This is crucial in the pharmaceutical industry where the stereochemistry of a molecule significantly impacts its biological activity.
Predicting the Product: Step-by-Step Approach
Predicting the product of a hydrogenation reaction involves a systematic approach:
-
Identify the Unsaturated Functional Group: The first step is to pinpoint the carbon-carbon double or triple bond present in the starting material.
-
Determine the Type of Hydrogenation: Based on the functional group, determine whether it's an alkene, alkyne, or aromatic hydrogenation.
-
Consider the Stereochemistry (for alkenes and alkynes): For alkenes, hydrogenation generally results in syn addition, meaning the two hydrogen atoms add to the same face of the double bond. For alkynes, the first hydrogenation step typically yields a cis-alkene.
-
Draw the Product: Add two hydrogen atoms across the multiple bond, ensuring the correct stereochemistry. Remember to adjust the number of bonds around each carbon atom to maintain tetrahedral geometry for saturated carbons.
-
Ignore Inorganic Byproducts: As instructed, we will ignore any inorganic byproducts (like water or the spent catalyst).
Examples: Drawing Products of Hydrogenation Reactions
Let's illustrate this with some examples:
Example 1: Hydrogenation of Propene
Starting material: Propene (CH₃CH=CH₂)
Reactants: H₂, Pd/C (palladium on carbon catalyst)
Product: Propane (CH₃CH₂CH₃)
Example 2: Hydrogenation of 2-butene
Starting material: (Z)-2-butene (CH₃CH=CHCH₃) (Note the Z-configuration indicating cis isomer)
Reactants: H₂, Pt
Product: Butane (CH₃CH₂CH₂CH₃) (The Z configuration is lost due to syn addition)
Example 3: Hydrogenation of 1-hexyne
Starting material: 1-hexyne (CH₃CH₂CH₂CH₂C≡CH)
Reactants: H₂, Lindlar's catalyst (a poisoned palladium catalyst that stops at the alkene stage)
Product: (Z)-1-hexene (CH₃CH₂CH₂CH₂CH=CH₂) (Lindlar's catalyst yields the cis-alkene)
Example 4: Hydrogenation of Benzene
Starting material: Benzene (C₆H₆)
Reactants: H₂, high pressure, Ni catalyst
Product: Cyclohexane (C₆H₁₂)
Example 5: Hydrogenation of a more complex alkene:
Let's consider a more complex alkene: (E)-3-methyl-2-pentene. This is shown below:
CH₃
|
CH₃CH₂C=CHCH₃
Upon hydrogenation with a typical catalyst like Pt or Pd/C, the hydrogen atoms will add across the double bond, resulting in:
CH₃
|
CH₃CH₂CHCH₂CH₃
This is 3-methylpentane. Note again, that the stereochemistry of the double bond is lost.
Example 6: Hydrogenation with Stereospecificity
Consider the hydrogenation of (E)-2-methyl-2-hexene with H2 and a metal catalyst. The product will be 2-methylhexane. The product will only have one structure because the double bond is not part of a ring and therefore is not subject to cis/trans isomerism.
Consider the hydrogenation of (Z)-2-methyl-2-hexene. The product remains 2-methylhexane. Note the absence of stereospecificity in this example.
Factors Affecting Hydrogenation
Several factors can influence the outcome of a hydrogenation reaction:
-
Catalyst: Different catalysts exhibit varying activities and selectivities. Some catalysts are more effective for alkene hydrogenation than alkyne hydrogenation, and some can control the stereochemistry of the product. Lindlar's catalyst is a prime example, promoting the formation of cis-alkenes from alkynes.
-
Pressure: Higher hydrogen pressures often lead to faster reaction rates.
-
Temperature: Higher temperatures generally increase the reaction rate, but can also lead to side reactions.
-
Solvent: The choice of solvent can influence the reaction rate and selectivity.
-
Steric Hindrance: Bulky substituents around the double bond can hinder the approach of the hydrogen molecule, slowing down the reaction or altering the regioselectivity.
Conclusion
Mastering the prediction of products in hydrogenation reactions is a fundamental skill for organic chemists. By understanding the reaction mechanism, considering the different types of hydrogenation, and accounting for the factors that influence reaction outcomes, one can accurately draw the product and predict the stereochemistry of the resulting molecule. This knowledge is crucial for synthetic chemists designing and executing complex organic synthesis pathways. Remember to always consider the catalyst and reaction conditions when predicting the product of any hydrogenation reaction. Practice is key! Work through various examples, and gradually you will develop a strong understanding of this essential reaction.
Latest Posts
Latest Posts
-
Domain 4 Lesson 2 Entrepreneurship And Small Business V 2
Mar 30, 2025
-
His 200 Module 7 Short Responses
Mar 30, 2025
-
Match Each Event With The Appropriate Stage Of Meiosis
Mar 30, 2025
-
What Is The Theme Of The Book Hatchet
Mar 30, 2025
-
The Souls Of Black Folk Chapter 1 Summary
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Draw The Product Of This Hydrogenation Reaction. Ignore Inorganic Byproducts . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
