Draw Two Resonance Structures Of The Cation Shown
Onlines
Mar 18, 2025 · 6 min read
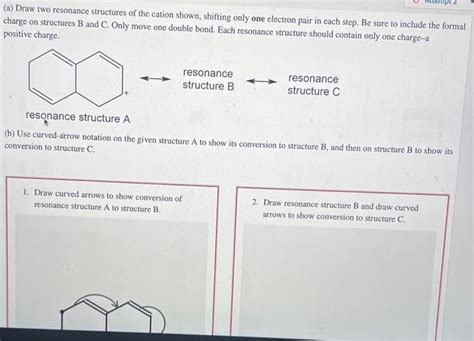
Table of Contents
Drawing Resonance Structures: A Deep Dive into the Cation's Delocalized Electrons
Resonance structures are a crucial concept in organic chemistry, representing the delocalization of electrons within a molecule or ion. They're particularly important when dealing with conjugated systems, where alternating single and multiple bonds allow for electron movement. This article will explore how to draw resonance structures, focusing specifically on a cation (positive ion) and providing a comprehensive understanding of the underlying principles. We'll delve into the intricacies of identifying conjugated systems, moving electrons, and evaluating the relative contribution of each resonance structure. Understanding resonance is key to predicting reactivity, stability, and other important properties of molecules.
What are Resonance Structures?
Before we tackle the specific cation, let's establish a solid foundation in resonance theory. Resonance structures are not different molecules; they are different representations of the same molecule or ion. A single Lewis structure often fails to fully capture the electron distribution in molecules with delocalized electrons. Resonance structures are used to show the different possible distributions of these electrons, giving a more complete and accurate picture of the molecule's true structure. The actual molecule is a hybrid of all the contributing resonance structures, a concept often referred to as the resonance hybrid. Think of it like this: the resonance structures are like different photographs of a chameleon – each shows a different color, but the chameleon itself is a blend of all those colors.
Identifying Conjugated Systems
The key to understanding resonance is recognizing conjugated systems. These systems contain alternating single and multiple bonds, often involving p-orbitals that can overlap to allow for electron delocalization. The crucial condition is the presence of a continuous chain of overlapping p-orbitals. This overlap allows electrons to move freely throughout the system. This is where things get interesting! A single Lewis structure cannot accurately depict the even distribution of electrons within such systems. This is where resonance structures become indispensable.
Examples of conjugated systems:
- Alternating C=C-C=C bonds: A classic example found in polyenes.
- C=O-C=C bonds: Common in carbonyl compounds with conjugated double bonds.
- Benzene rings: The quintessential example of a highly delocalized system.
Drawing Resonance Structures: A Step-by-Step Guide
Now let's proceed to the actual drawing of resonance structures. The process involves systematically moving electrons within the conjugated system, keeping in mind several key rules:
-
Identify the conjugated system: Pinpoint the portion of the molecule with alternating single and multiple bonds and overlapping p-orbitals.
-
Move electrons in pairs: Electrons are moved, not atoms. Always move electrons in pairs (lone pairs or π electrons) using curved arrows. These arrows show the movement of electron density.
-
Maintain the same number of valence electrons: The total number of valence electrons should remain constant in all resonance structures. No electrons are gained or lost.
-
Respect the octet rule (mostly): While exceptions exist, especially with larger atoms, strive to maintain a full octet for atoms in the second row (carbon, nitrogen, oxygen, etc.).
-
Formal charges: Keep track of formal charges. Moving electrons can alter the formal charges of atoms.
-
Draw all possible resonance structures: Don't miss any valid structures that contribute to the resonance hybrid.
Example: Drawing Resonance Structures of a Cation
Let's imagine we have a cation (a positively charged ion) with a conjugated system. While a specific cation isn't provided in the prompt, we'll create a hypothetical example to illustrate the process. Let’s consider a carbocation with a conjugated double bond system. This allows us to demonstrate the principles of resonance for a common type of cation found in many organic reactions.
Let's say our cation looks like this:
+
|
CH2=CH-CH2
Step 1: Identify the Conjugated System: The conjugated system involves the carbon-carbon double bond and the empty p-orbital on the positively charged carbon atom. The positive charge indicates a deficiency of electrons.
Step 2: Draw Resonance Structures:
We can move a pair of electrons from the double bond to form a new double bond with the positively charged carbon. This will result in the positive charge moving to another carbon atom.
Resonance Structure 1 (Original):
+
|
CH2=CH-CH2
Resonance Structure 2:
CH2-CH=CH2
+
In Resonance Structure 2, the positive charge has moved to the end carbon atom. Both structures show the delocalization of the positive charge across the conjugated system.
Step 3: Evaluate the Contribution of Each Structure: This involves analyzing the stability of each resonance structure. Factors that increase stability include:
- Complete octets: Structures with complete octets on all atoms are generally more stable.
- Minimizing formal charges: Structures with fewer formal charges or smaller magnitudes of charges are more stable.
- Placing negative charges on more electronegative atoms: If negative charges are present, they are more stable on electronegative atoms.
- Placing positive charges on less electronegative atoms: If positive charges are present, they are more stable on less electronegative atoms.
In our example, both structures have incomplete octets on the positively charged carbon. However, Resonance Structure 1 and Resonance Structure 2 contribute equally to the overall resonance hybrid.
Advanced Concepts and Considerations
-
Resonance Energy: The resonance hybrid is more stable than any of its individual resonance structures. The difference in energy between the resonance hybrid and the most stable contributing resonance structure is termed resonance energy. This extra stability arises from the delocalization of electrons.
-
Major and Minor Contributors: In some cases, certain resonance structures contribute more significantly to the resonance hybrid than others. These are called major contributors. Structures with less favorable features (e.g., high formal charges, incomplete octets on electronegative atoms) are minor contributors.
-
Aromatic Systems: Aromatic compounds, like benzene, exhibit a particularly high degree of resonance stabilization due to their cyclic, conjugated, and planar structure fulfilling Hückel's rule (4n+2 π electrons).
-
Limitations of Resonance Structures: Resonance structures are a model; they don't represent the actual structure of the molecule, which is the resonance hybrid. They are a tool to better understand electron delocalization and predict molecular properties.
Conclusion: Mastering Resonance Structures
Understanding and drawing resonance structures is crucial for mastering organic chemistry. The ability to identify conjugated systems, systematically move electrons, and evaluate the relative contributions of different resonance structures is essential for predicting reactivity, stability, and other important molecular properties. By carefully following the steps outlined in this guide and practicing with various examples, you will confidently tackle the complexities of resonance and deepen your understanding of chemical bonding. Remember that the key is to visualize the delocalized electrons and their movement within the molecule's framework. The more you practice, the more intuitive this process will become. Continue exploring more complex molecules and challenging resonance problems to solidify your skills and enhance your comprehension of this fundamental aspect of chemistry. The ability to effectively utilize resonance theory is a testament to your growing expertise in the field of organic chemistry.
Latest Posts
Latest Posts
-
A Lesson Before Dying Chapter Summary
Mar 18, 2025
-
And Then There Were None Quotes
Mar 18, 2025
-
Https Www Raterhub Com Evaluation Rater
Mar 18, 2025
-
1 4 Additional Practice Literal Equations And Formulas
Mar 18, 2025
-
7 3 Additional Practice Proving Triangles Similar
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Draw Two Resonance Structures Of The Cation Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
