Experiment 3 Osmosis Direction And Concentration Gradients
Onlines
Mar 15, 2025 · 6 min read
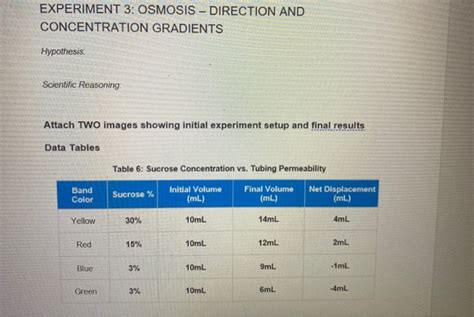
Table of Contents
Experiment 3: Osmosis, Direction, and Concentration Gradients: A Deep Dive
Understanding osmosis is crucial in biology, chemistry, and numerous applied sciences. This detailed exploration of Experiment 3 focuses on the principles governing osmosis, specifically its direction and the influence of concentration gradients. We'll delve into the experimental setup, expected results, potential sources of error, and real-world applications.
Understanding Osmosis: A Fundamental Biological Process
Osmosis is the passive movement of water molecules across a selectively permeable membrane from a region of high water concentration to a region of low water concentration. This movement continues until equilibrium is reached, meaning the water concentration is equal on both sides of the membrane. Crucially, this movement is driven by the difference in water potential, not by a direct expenditure of energy. The selectively permeable membrane, often a cell membrane, allows water molecules to pass through but restricts the movement of larger solutes.
Key Terms:
- Selectively Permeable Membrane: A membrane that allows certain molecules to pass through while blocking others. Cell membranes are prime examples.
- Water Potential: The tendency of water to move from one area to another. Pure water has the highest water potential. The addition of solutes lowers the water potential.
- Concentration Gradient: The difference in solute concentration between two areas. Osmosis is driven by the concentration gradient of water, moving from high concentration (high water potential) to low concentration (low water potential).
- Isotonic Solution: A solution with the same solute concentration as the cell's cytoplasm. No net movement of water occurs.
- Hypotonic Solution: A solution with a lower solute concentration than the cell's cytoplasm. Water moves into the cell, potentially causing it to swell or burst (lyse).
- Hypertonic Solution: A solution with a higher solute concentration than the cell's cytoplasm. Water moves out of the cell, causing it to shrink (crenate).
Experiment 3 Setup: Investigating Osmosis in Action
A typical Experiment 3 setup involves using a selectively permeable membrane, often dialysis tubing, to separate two solutions of differing solute concentrations. The dialysis tubing mimics a cell membrane. One side contains a solution with a high concentration of solute (e.g., sucrose), while the other side contains a solution with a low concentration of solute (or pure water). The change in weight or volume of either solution over time is measured to determine the direction and rate of water movement.
Materials:
- Dialysis tubing
- Sucrose solutions of varying concentrations (e.g., 0%, 10%, 20%)
- Distilled water
- Beakers
- Graduated cylinders
- Weighing scale
- Timer
Procedure:
- Prepare the dialysis tubing: Soak the dialysis tubing in water to make it pliable.
- Create the experimental bags: Fill sections of the dialysis tubing with different sucrose solutions, ensuring no air is trapped. Tie off the ends securely.
- Weigh the bags: Carefully weigh each bag to establish a baseline.
- Immerse the bags: Place each bag into a separate beaker filled with distilled water.
- Monitor and record data: At regular intervals (e.g., every 15 minutes for 1-2 hours), remove the bags, blot them gently, and weigh them again. Record the weight changes.
Expected Results and Interpretation
The expected results are directly related to the concentration gradients.
- Bags with higher sucrose concentration: These bags will lose water to the surrounding distilled water. Their weight will decrease over time. This is because the water potential inside the bag is lower (due to the high solute concentration) than the water potential outside the bag.
- Bags with lower sucrose concentration (or distilled water): These bags will gain water from the surrounding solution. Their weight will increase. This occurs because the water potential inside the bag is higher than the water potential outside.
By plotting the weight change over time for each bag, you can visualize the rate of osmosis. A steeper slope indicates a faster rate of water movement. The difference in weight change between bags with different sucrose concentrations demonstrates the direct relationship between concentration gradient and the rate of osmosis. The larger the concentration difference, the faster the rate of osmosis.
Sources of Error and Experimental Refinements
Several factors can influence the accuracy of Experiment 3. Understanding and mitigating these errors is crucial for reliable results.
Potential Sources of Error:
- Membrane leakage: Imperfect seals in the dialysis tubing can lead to solute leakage, affecting the concentration gradient and thus the osmosis rate.
- Evaporation: Water loss due to evaporation from the beakers can skew the weight measurements.
- Temperature fluctuations: Changes in temperature can affect the rate of osmosis. Maintaining a consistent temperature throughout the experiment is essential.
- Inconsistent bag sizes: Using dialysis tubing of varying sizes can introduce variability in the surface area available for osmosis, leading to inconsistent results.
- Incomplete equilibration: Not allowing sufficient time for equilibrium to be reached will result in inaccurate measurements of the final weight or volume.
Refinements for Improved Accuracy:
- Use high-quality dialysis tubing with minimal defects.
- Seal the dialysis tubing meticulously to prevent leakage.
- Conduct the experiment in a controlled environment with consistent temperature.
- Use bags of similar size and weight.
- Use multiple replicates for each sucrose concentration to reduce the impact of random error.
- Regularly check and control the water level in the beakers.
Real-World Applications of Osmosis
Osmosis isn't just a laboratory phenomenon; it's a fundamental process with widespread applications across diverse fields.
Biology and Medicine:
- Water uptake by plants: Osmosis plays a critical role in water absorption by plant roots. Water moves from the soil (high water potential) into the root cells (lower water potential).
- Kidney function: The kidneys use osmosis to filter waste products from the blood and regulate water balance.
- Cell volume regulation: Cells maintain their volume through osmotic balance, preventing swelling or shrinking.
- Drug delivery: Osmosis is exploited in certain drug delivery systems to control the release of medication.
- Understanding dehydration and hydration: Osmosis explains why dehydration leads to cell shrinkage and why excessive water intake can cause cell swelling.
Food Science and Technology:
- Food preservation: Osmosis is employed in techniques like osmosis dehydration to remove water from food, increasing shelf life.
- Fruit and vegetable processing: Understanding osmosis helps optimize the processes for preserving texture and flavor during food processing.
Environmental Science:
- Water purification: Reverse osmosis, a process that utilizes pressure to overcome osmotic pressure, is used for water purification.
- Salinity control in agriculture: Understanding osmotic pressure helps manage salinity levels in irrigated lands.
Other Applications:
- Industrial processes: Osmosis is involved in various industrial processes, such as membrane separation techniques.
- Analytical chemistry: Osmotic pressure measurements can help determine molecular weight of polymers.
Conclusion: Expanding Your Understanding of Osmosis
Experiment 3 offers a hands-on approach to understanding the principles governing osmosis, the influence of concentration gradients, and the real-world implications of this fundamental process. By meticulously following the experimental procedure, carefully analyzing results, and understanding potential sources of error, you can gain a deeper appreciation for the importance of osmosis in biological, chemical, and environmental systems. Remember to always prioritize accuracy and safety during your experimentation. Through careful observation and data analysis, you can further enhance your grasp of osmosis and its significance in various scientific domains. Furthermore, exploring related concepts such as tonicity and water potential will significantly broaden your understanding of this crucial biological process.
Latest Posts
Latest Posts
-
Excerpts From Romeo And Juliet Commonlit Answers
Mar 17, 2025
-
Tuesdays With Morrie Summary Per Chapter
Mar 17, 2025
-
Esta Manana Comi Frutas En El
Mar 17, 2025
-
10 2 3 Select And Configure Dual Monitors
Mar 17, 2025
-
The Dutch Hunger Winter Case Study Answers
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Experiment 3 Osmosis Direction And Concentration Gradients . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
