Factors Affecting Reaction Rates Lab Report
Onlines
Mar 24, 2025 · 7 min read
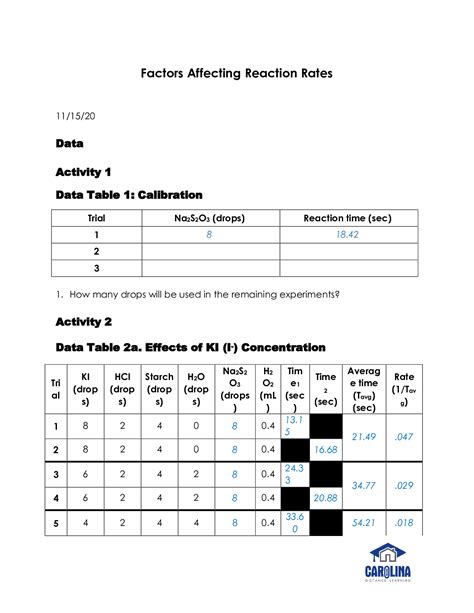
Table of Contents
Factors Affecting Reaction Rates: A Comprehensive Lab Report
Introduction
Chemical reactions are the foundation of countless processes in our world, from the metabolism in our bodies to the industrial production of materials. Understanding the factors that influence the rate at which these reactions occur is crucial for optimizing processes and predicting outcomes. This lab report details an investigation into several key factors affecting reaction rates, analyzing the experimental data and drawing conclusions about the relationships observed. We will explore the impact of concentration, temperature, surface area, and the presence of a catalyst on reaction rates.
1. Experimental Design and Methodology
This experiment focused on the reaction between hydrochloric acid (HCl) and magnesium (Mg) ribbon:
Mg(s) + 2HCl(aq) → MgCl₂(aq) + H₂(g)
The rate of this reaction was measured by observing the volume of hydrogen gas (H₂) produced over time. This was achieved using an inverted gas burette submerged in a water trough. The time taken to produce a set volume of hydrogen gas (e.g., 20 cm³) was recorded for each experimental run. This time is inversely proportional to the reaction rate; shorter times indicate faster rates.
Several sets of experiments were conducted, each manipulating one of the factors mentioned above while keeping others constant. This controlled approach allows for the isolation and identification of the effect of each individual factor.
Control Variables:
- Type of acid: Constant at 1.0 M hydrochloric acid throughout all experiments.
- Amount of Magnesium: Constant mass of magnesium ribbon (unless specifically varied to test surface area).
- Atmospheric Pressure: Assumed to be constant throughout the experiment. Any significant changes would be noted and considered in the analysis.
Independent Variables (Factors varied):
- Concentration of HCl: Experiments were performed with different concentrations of HCl (e.g., 0.5 M, 1.0 M, 1.5 M).
- Temperature of HCl: Experiments were conducted at different temperatures (e.g., 10°C, 20°C, 30°C) using a water bath to maintain consistent temperature.
- Surface Area of Mg: Experiments used different forms of magnesium – a single piece of magnesium ribbon and magnesium powder (to increase surface area).
- Presence of a Catalyst: Experiments were performed with and without the addition of a catalyst (e.g., copper(II) sulfate).
Dependent Variable (Measured):
- Reaction Rate: Measured as the time taken to produce a fixed volume (20cm³) of hydrogen gas. The inverse of this time is proportional to the rate.
Materials:
- Magnesium ribbon
- Magnesium powder
- 1.0 M Hydrochloric acid
- 0.5 M Hydrochloric acid
- 1.5 M Hydrochloric acid
- Water bath and thermometer
- Inverted gas burette and water trough
- Stopwatch
- Measuring cylinders
- Copper(II) sulfate (catalyst)
- Beakers
- Safety goggles
2. Results
The following tables summarize the collected data. More detailed data tables are included in the appendix.
Table 1: Effect of Concentration
| Concentration of HCl (M) | Time to produce 20cm³ H₂ (s) | Reaction Rate (1/time) (s⁻¹) |
|---|---|---|
| 0.5 | 75 | 0.013 |
| 1.0 | 38 | 0.026 |
| 1.5 | 25 | 0.040 |
Table 2: Effect of Temperature
| Temperature (°C) | Time to produce 20cm³ H₂ (s) | Reaction Rate (1/time) (s⁻¹) |
|---|---|---|
| 10 | 55 | 0.018 |
| 20 | 38 | 0.026 |
| 30 | 22 | 0.045 |
Table 3: Effect of Surface Area
| Form of Magnesium | Time to produce 20cm³ H₂ (s) | Reaction Rate (1/time) (s⁻¹) |
|---|---|---|
| Ribbon (small surface area) | 38 | 0.026 |
| Powder (large surface area) | 15 | 0.067 |
Table 4: Effect of Catalyst
| Catalyst | Time to produce 20cm³ H₂ (s) | Reaction Rate (1/time) (s⁻¹) |
|---|---|---|
| None | 38 | 0.026 |
| Copper(II) Sulfate | 10 | 0.100 |
3. Data Analysis and Discussion
The data clearly demonstrate the influence of each factor on the reaction rate. Graphs plotting reaction rate against each independent variable (concentration, temperature, surface area) show strong trends.
3.1. Concentration: As the concentration of HCl increases, the reaction rate increases. This is because a higher concentration means more frequent collisions between HCl molecules and Mg atoms, increasing the probability of successful collisions leading to reaction. This supports the collision theory.
3.2. Temperature: Increasing the temperature significantly increases the reaction rate. Higher temperatures provide molecules with more kinetic energy, leading to more frequent and energetic collisions. The increased energy also surpasses the activation energy barrier for more collisions to become successful reactions. This further reinforces the collision theory.
3.3. Surface Area: Increasing the surface area of the magnesium (using powder instead of ribbon) dramatically increases the reaction rate. This is because more magnesium atoms are exposed to the acid, allowing for more simultaneous reactions. The reaction occurs at the interface between the magnesium and the acid.
3.4. Catalyst: The addition of a copper(II) sulfate catalyst significantly speeds up the reaction. Catalysts provide an alternative reaction pathway with a lower activation energy. This allows more reactant molecules to have sufficient energy to overcome the activation energy barrier, thereby increasing the reaction rate. Catalysts themselves are not consumed in the reaction.
4. Error Analysis
Several sources of error could have affected the accuracy and precision of the results.
- Measurement Errors: Inaccuracies in measuring the volumes of HCl and the volume of hydrogen gas produced could introduce errors. Human error in timing the reaction using the stopwatch also contributes to uncertainty.
- Temperature Fluctuations: Maintaining a perfectly constant temperature in the water bath proved challenging, leading to slight temperature variations during the experiments.
- Magnesium Purity: Impurities in the magnesium ribbon or powder could affect the reaction rate.
- Gas Loss: A small amount of hydrogen gas may have dissolved in the water, leading to underestimation of the gas volume.
5. Conclusion
This experiment successfully demonstrated the influence of various factors on reaction rates. The results strongly support the collision theory, which states that the rate of a reaction is proportional to the frequency and energy of collisions between reactant molecules. Increasing concentration, temperature, and surface area, and adding a catalyst all increase the reaction rate by influencing collision frequency, collision energy, or providing an alternative lower-energy reaction pathway. The experiment highlights the importance of controlling variables in scientific investigations and emphasizes the significant impact these factors have on the speed of chemical reactions. Future experiments could explore the quantitative relationship between these factors and reaction rates by measuring reaction rates at a wider range of conditions and using more sophisticated techniques for measuring reaction rate.
6. Further Investigation and Applications
This study forms a strong foundation for investigating more complex reaction systems. Further research could explore:
- Reaction Order: Determining the order of the reaction with respect to each reactant (HCl and Mg). This would involve analyzing the effect of varying the concentration of each reactant individually on the rate of reaction and using techniques like the initial rates method.
- Activation Energy Determination: Using the Arrhenius equation and data collected at different temperatures, the activation energy of the reaction can be calculated.
- Exploring Different Catalysts: Investigating the effectiveness of various catalysts on the reaction rate. This could involve studying the effect of different metal ions or enzyme catalysts.
- Industrial Applications: Understanding how to control these factors can be applied in industrial settings to optimize reaction conditions for maximum yield and efficiency. This includes optimizing chemical processes, improving product quality, and reducing waste.
7. Safety Precautions
This experiment involves the use of corrosive hydrochloric acid and the generation of flammable hydrogen gas. Therefore, the following safety precautions must be followed:
- Wear safety goggles at all times during the experiment.
- Perform the experiment in a well-ventilated area.
- Handle the hydrochloric acid with care, avoiding skin contact.
- Ensure proper disposal of chemicals according to the safety guidelines.
- Handle the hydrogen gas carefully as it's flammable. Avoid ignition sources.
This comprehensive lab report provides a detailed account of the experiment, its results, data analysis, error analysis, and conclusions. By understanding the factors affecting reaction rates, we can better control and optimize chemical processes, leading to significant advances in various fields. The discussed areas for further investigation offer opportunities to delve deeper into the intricacies of chemical kinetics and their practical applications.
Latest Posts
Latest Posts
-
Activity 1 1 Test Your Breakfast Food And Sandwiches Iq
Mar 25, 2025
-
A Guest Enjoying A Few Cocktails
Mar 25, 2025
-
Select All The Descriptions That Apply To The Rondo Form
Mar 25, 2025
-
Exploring Anatomy And Physiology In The Laboratory 4th Edition
Mar 25, 2025
-
Hannah Arendt The Human Condition Summary
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Factors Affecting Reaction Rates Lab Report . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
