For The Solutions That You Will Prepare In Step 2
Onlines
Mar 16, 2025 · 6 min read
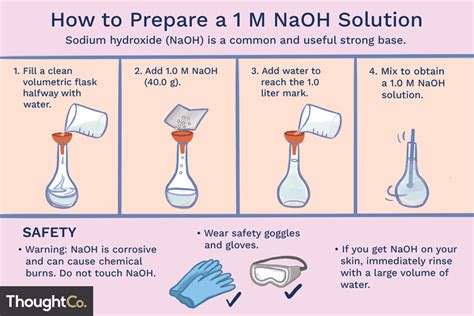
Table of Contents
Preparing Solutions for Analytical Chemistry: A Comprehensive Guide
Step 2 in any analytical chemistry experiment usually involves preparing solutions. This process, seemingly straightforward, is critical for accurate and reliable results. A poorly prepared solution can lead to significant errors, invalidating the entire experiment. This article delves into the detailed procedures, considerations, and best practices for preparing solutions of various types, ensuring accurate and reproducible results in your analytical work.
Understanding Solution Preparation Fundamentals
Before diving into specific solution preparation techniques, understanding the fundamental concepts is crucial. This section will cover essential terminology and principles that underpin the entire process.
1. Concentration Units: Accurate solution preparation requires a clear understanding of concentration units. The most common include:
- Molarity (M): Moles of solute per liter of solution. This is arguably the most widely used concentration unit in analytical chemistry.
- Molality (m): Moles of solute per kilogram of solvent. Molality is less affected by temperature changes compared to molarity.
- Normality (N): Equivalents of solute per liter of solution. This unit is less frequently used now, but still relevant in certain applications like acid-base titrations.
- Percent by Weight (% w/w): Grams of solute per 100 grams of solution.
- Percent by Volume (% v/v): Milliliters of solute per 100 milliliters of solution. Commonly used for liquid-liquid solutions.
- Parts per Million (ppm) and Parts per Billion (ppb): These units express the concentration of a solute in very dilute solutions. 1 ppm = 1 mg/L and 1 ppb = 1 µg/L.
2. Standard Solutions: These are solutions of precisely known concentration, often prepared from a primary standard – a highly pure substance with a known chemical formula and stable, non-hygroscopic properties (meaning they don't absorb water from the air). Standard solutions are essential for titrations and other quantitative analytical methods.
3. Volumetric Flasks: The cornerstone of accurate solution preparation, volumetric flasks are calibrated to contain a precise volume of liquid at a specified temperature (usually 20°C). Their accuracy is crucial for ensuring the correct concentration of the prepared solution.
4. Pipettes and Beakers: While volumetric flasks provide the final volume, pipettes and beakers play crucial roles in transferring and diluting solutions. Pipettes are used for delivering precise volumes, while beakers are generally used for less precise measurements and mixing.
5. Water Purity: The purity of the water used is paramount. Distilled water or deionized water is generally required, and in some cases, ultrapure water is necessary to avoid interference from dissolved impurities.
Preparing Solutions from Solids
This section details the steps for preparing solutions from solid solutes, a common task in analytical chemistry.
1. Calculation: First, accurately calculate the required mass of solute needed to achieve the desired concentration and volume. Using the formula for molarity (M = moles/liter) or other relevant concentration units is crucial here. Consider the molar mass of the solute to convert between mass and moles. A simple example: to prepare 100 mL of a 1 M NaCl solution, the following calculation is necessary:
- Molar mass of NaCl ≈ 58.44 g/mol
- Moles of NaCl = Molarity × Volume (in Liters) = 1 M × 0.1 L = 0.1 moles
- Mass of NaCl = Moles × Molar mass = 0.1 moles × 58.44 g/mol ≈ 5.844 g
2. Weighing: Using an analytical balance, weigh the calculated mass of the solid solute accurately. The balance's accuracy directly impacts the final solution's accuracy. Ensure the balance is properly calibrated and tared before weighing. Handle the solid carefully to avoid contamination.
3. Dissolution: Transfer the weighed solute into a clean beaker. Add a small amount of the chosen solvent (usually water) and stir gently to dissolve the solid completely. For some solutes, heating might be necessary, but this should be done carefully to avoid loss of solute or solvent. Ensure complete dissolution before proceeding to the next step.
4. Transfer to Volumetric Flask: Carefully transfer the dissolved solute from the beaker into a clean, dry volumetric flask of the desired volume. Use a funnel to minimize spillage. Rinse the beaker thoroughly with small portions of the solvent and add these rinsings to the volumetric flask to ensure complete transfer of the solute.
5. Dilution to Mark: Add solvent to the volumetric flask until the bottom of the meniscus is at the etched mark on the neck of the flask. Ensure the solution is thoroughly mixed by gently inverting the flask several times.
6. Mixing and Storage: After thorough mixing, carefully label the flask with the name of the solution, its concentration, the date of preparation, and any other relevant information. Store the solution appropriately, depending on its properties (e.g., in a dark, cool place for light-sensitive solutions).
Preparing Solutions from Concentrated Solutions
Preparing solutions by diluting existing concentrated solutions (like stock solutions) is a common practice to save time and resources.
1. Dilution Calculation: Use the dilution formula, M1V1 = M2V2, where M1 is the initial concentration, V1 is the initial volume, M2 is the final concentration, and V2 is the final volume. Solve for the required volume (V1) of the concentrated solution.
2. Pipetting: Using a pipette, carefully measure the calculated volume (V1) of the concentrated solution. Pipettes provide accurate volume measurements, critical for accurate dilution.
3. Transfer and Dilution: Transfer the measured volume of the concentrated solution to a volumetric flask of the desired final volume (V2). Add solvent to the flask, gradually and carefully, while mixing gently. Avoid splashing.
4. Dilution to Mark: Once the solution is thoroughly mixed, carefully add solvent until the meniscus reaches the mark on the volumetric flask’s neck.
5. Mixing and Storage: Mix the solution thoroughly by inverting the flask several times. Label the flask properly, indicating the solution's name, concentration, date of preparation, and other relevant information. Store the solution as necessary, following any special handling instructions.
Special Considerations for Specific Solutions
Some solutions require additional considerations during preparation.
1. Acidic and Basic Solutions: Always add acid to water, never water to acid. Adding water to acid can cause a violent exothermic reaction, potentially leading to splashing and burns.
2. Light-Sensitive Solutions: Store these solutions in amber or dark-colored bottles to prevent degradation from light exposure.
3. Volatile Solutions: Handle volatile solutions in a well-ventilated area to minimize the risk of inhalation.
4. Hygroscopic Solutions: Handle hygroscopic solutions quickly and efficiently to minimize the absorption of atmospheric moisture, which can alter the concentration.
5. Oxidizing Agents: Handle oxidizing agents with extreme care, as they can be reactive and potentially dangerous.
Error Analysis and Quality Control
Accuracy is paramount in solution preparation. Several steps ensure quality control:
1. Calibration of Equipment: Ensure all glassware is properly calibrated to minimize measurement errors.
2. Repeatability: Prepare multiple solutions and compare their concentrations to assess repeatability.
3. Standardisation: For many analytical applications, especially titrations, the prepared solution should be standardized against a primary standard to determine its exact concentration.
Conclusion
Preparing solutions accurately and efficiently is a foundational skill in analytical chemistry. By carefully following the steps outlined in this guide, paying close attention to detail, and understanding the principles behind solution preparation, you can ensure the accuracy and reliability of your experimental results. Remember that meticulous record-keeping is essential for traceability and repeatability. Consistent practice and attention to detail will refine your technique, leading to improved experimental outcomes and enhanced confidence in your analytical work. The quality of your solutions directly reflects the quality of your data. Always strive for accuracy and precision in this crucial step of your analytical process.
Latest Posts
Latest Posts
-
Summary Of Chapter 5 Of The Giver
Mar 16, 2025
-
European And American Indian First Encounters Dbq
Mar 16, 2025
-
Covey Matrix Eight Dimensions Of Wellness
Mar 16, 2025
-
Color By Number Photosynthesis Answer Key
Mar 16, 2025
-
Label The Floors Of The Hotel
Mar 16, 2025
Related Post
Thank you for visiting our website which covers about For The Solutions That You Will Prepare In Step 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
