Isotopes Ions And Atoms Worksheet 2
Onlines
Mar 13, 2025 · 7 min read
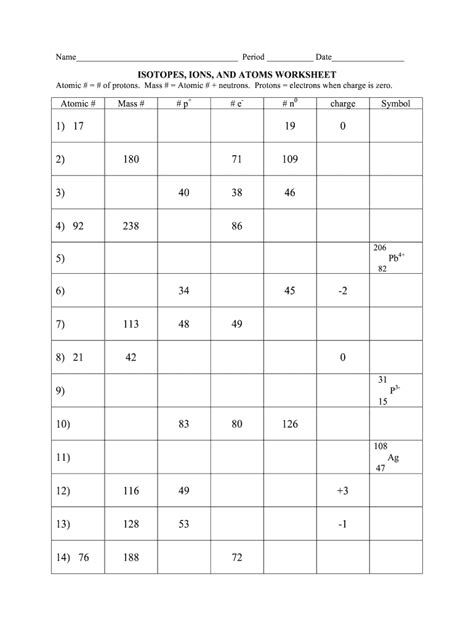
Table of Contents
Isotopes, Ions, and Atoms: Worksheet 2 Deep Dive
This comprehensive guide delves into the concepts of isotopes, ions, and atoms, providing a detailed explanation to complement your Worksheet 2. We'll explore the fundamental differences and similarities between these subatomic entities, clarifying common misconceptions and building a strong foundation in atomic structure.
Understanding the Building Blocks: Atoms
Before diving into isotopes and ions, let's solidify our understanding of the atom itself. An atom is the basic unit of a chemical element. It's the smallest particle that retains the chemical properties of that element. Atoms are incredibly tiny, far too small to be seen with the naked eye, even with the most powerful optical microscopes. Each atom consists of a central nucleus, containing positively charged protons and electrically neutral neutrons. Surrounding the nucleus is a cloud of negatively charged electrons.
Key Characteristics of Atoms:
- Atomic Number (Z): This represents the number of protons in an atom's nucleus. It uniquely identifies the element. For example, all atoms with an atomic number of 6 are carbon atoms.
- Mass Number (A): This is the total number of protons and neutrons in the atom's nucleus. It represents the approximate mass of the atom.
- Electron Configuration: This describes how the electrons are arranged in energy levels or shells around the nucleus. Electron configuration determines the chemical behavior of an atom.
Isotopes: Variations on a Theme
Isotopes are atoms of the same element that have the same atomic number (same number of protons) but differ in their mass number (different number of neutrons). Since they have the same number of protons, isotopes have the same chemical properties. However, their physical properties, such as density and radioactivity, can differ slightly due to the differing neutron numbers.
Understanding Isotopic Notation:
Isotopes are often represented using isotopic notation. This notation includes the element symbol, the mass number (A) as a superscript, and the atomic number (Z) as a subscript. For example:
- ¹²C (Carbon-12): This represents a carbon atom with 6 protons and 6 neutrons (12 - 6 = 6 neutrons).
- ¹⁴C (Carbon-14): This represents a carbon atom with 6 protons and 8 neutrons (14 - 6 = 8 neutrons).
Both ¹²C and ¹⁴C are isotopes of carbon. ¹⁴C is a radioactive isotope, meaning its nucleus is unstable and decays over time, emitting radiation. This property is crucial in carbon dating techniques used to determine the age of ancient artifacts.
Examples of Isotopes and Their Applications:
- Uranium (U): Uranium has several isotopes, including ²³⁵U and ²³⁸U. ²³⁵U is fissile, meaning it can sustain a nuclear chain reaction and is used in nuclear power plants and weapons. ²³⁸U is not fissile but can be converted into plutonium, which is also fissile.
- Hydrogen (H): Hydrogen has three isotopes: ¹H (protium), ²H (deuterium), and ³H (tritium). Deuterium and tritium are heavier isotopes of hydrogen, with deuterium being stable and tritium being radioactive. Deuterium is used in nuclear fusion research and heavy water reactors.
- Carbon (C): As mentioned before, ¹⁴C is crucial in radiocarbon dating. The ratio of ¹⁴C to ¹²C in organic materials can be used to estimate their age.
Ions: Charged Particles
Unlike isotopes, ions are atoms or molecules that have gained or lost one or more electrons. This results in a net electrical charge. If an atom loses electrons, it becomes a positively charged ion called a cation. If an atom gains electrons, it becomes a negatively charged ion called an anion.
Ion Formation:
Ion formation occurs due to the transfer of electrons between atoms. This transfer is driven by the tendency of atoms to achieve a stable electron configuration, often a full outermost electron shell (octet rule). Atoms with a strong tendency to lose electrons become cations, while those with a strong tendency to gain electrons become anions.
Representing Ions:
Ions are represented by the element symbol followed by the charge as a superscript. For example:
- Na⁺ (Sodium ion): A sodium atom loses one electron, resulting in a +1 charge.
- Cl⁻ (Chloride ion): A chlorine atom gains one electron, resulting in a -1 charge.
- Ca²⁺ (Calcium ion): A calcium atom loses two electrons, resulting in a +2 charge.
- O²⁻ (Oxide ion): An oxygen atom gains two electrons, resulting in a -2 charge.
The Role of Ions in Chemical Bonding:
Ions play a crucial role in ionic bonding, a type of chemical bond formed by the electrostatic attraction between oppositely charged ions. This type of bonding is responsible for the formation of many crystalline solids, such as sodium chloride (table salt).
Distinguishing Between Isotopes and Ions:
It's crucial to distinguish between isotopes and ions. While isotopes differ in the number of neutrons, ions differ in the number of electrons. Isotopes are electrically neutral, while ions carry a net electrical charge. Isotopes are variations of the same element, maintaining their chemical identity, whereas ions are charged forms of atoms or molecules, often exhibiting different chemical behavior.
Worksheet 2 Practice Problems: A Deeper Dive
Let's delve into some hypothetical problems similar to those found in Worksheet 2, using the knowledge we've gained.
Problem 1: An atom has an atomic number of 17 and a mass number of 35. Identify the element and determine the number of protons, neutrons, and electrons in a neutral atom of this element.
Solution:
- The atomic number (17) identifies the element as Chlorine (Cl).
- The number of protons is equal to the atomic number, which is 17.
- The number of neutrons is the mass number minus the atomic number: 35 - 17 = 18 neutrons.
- In a neutral atom, the number of electrons is equal to the number of protons: 17 electrons.
Problem 2: Compare and contrast the isotopes ¹⁶O and ¹⁸O.
Solution:
Both ¹⁶O and ¹⁸O are isotopes of oxygen. They have the same number of protons (8) but differ in the number of neutrons:
- ¹⁶O has 8 neutrons (16 - 8 = 8).
- ¹⁸O has 10 neutrons (18 - 8 = 10).
They have the same chemical properties due to the same number of protons and electrons but may have slightly different physical properties.
Problem 3: Explain the formation of the magnesium ion, Mg²⁺.
Solution:
Magnesium (Mg) has an atomic number of 12, meaning it has 12 protons and 12 electrons in its neutral state. To achieve a stable electron configuration (like that of Neon), magnesium readily loses two electrons from its outermost shell. This loss results in a +2 charge, forming the Mg²⁺ cation.
Problem 4: Describe the difference between an atom of sodium (Na) and a sodium ion (Na⁺).
Solution:
A sodium atom (Na) has 11 protons and 11 electrons, resulting in a neutral charge. A sodium ion (Na⁺) has 11 protons but only 10 electrons, resulting in a +1 charge due to the loss of one electron. The chemical properties of the sodium atom and the sodium ion are significantly different.
Problem 5: A certain ion has 10 protons, 10 neutrons, and 8 electrons. Identify the ion and its charge.
Solution:
- 10 protons identify the element as Neon (Ne).
- 10 neutrons contribute to the mass number but do not affect the charge.
- With 10 protons and 8 electrons, the ion has a net charge of +2. Therefore, the ion is Ne²⁺.
These examples demonstrate the application of the concepts discussed. By working through these problems and similar ones in Worksheet 2, you will solidify your understanding of isotopes, ions, and atoms. Remember to carefully consider the numbers of protons, neutrons, and electrons in each scenario to determine the identity and charge of the particle.
Expanding Your Knowledge: Further Exploration
This in-depth analysis should provide you with a robust understanding of isotopes, ions, and atoms. To further enhance your comprehension, consider exploring the following topics:
- Radioactive decay: Investigate the different types of radioactive decay (alpha, beta, gamma) and their applications.
- Nuclear reactions: Learn about nuclear fission and fusion and their implications for energy production.
- Ionic compounds: Explore the properties and structures of ionic compounds formed through the interaction of cations and anions.
- Advanced isotopic notation: Explore how isotopes are used to trace chemical processes and reactions in various fields.
By continuing to explore these related concepts, you can build a comprehensive and nuanced understanding of the fundamental building blocks of matter. Remember that consistent practice and exploration are key to mastering this essential aspect of chemistry. Good luck with Worksheet 2 and your continued studies!
Latest Posts
Latest Posts
-
Separation Of The Components Of A Mixture Pre Lab Answers
Mar 14, 2025
-
The Inzinzebu Bandits Are Harassing The Good Merchants In
Mar 14, 2025
-
Introduction To Acids And Bases Webquest Answer Key
Mar 14, 2025
-
A Newly Formed From Must Decide
Mar 14, 2025
-
Foundations In Health And Safety E Learning Post Test Answers
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Isotopes Ions And Atoms Worksheet 2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
