Label Each Carbon Atom With The Appropriate Geometry
Onlines
Mar 24, 2025 · 6 min read
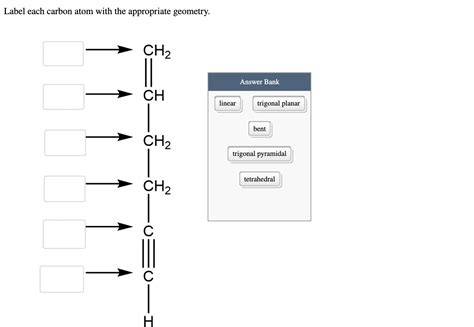
Table of Contents
Labeling Carbon Atoms: A Comprehensive Guide to Molecular Geometry
Determining the geometry around each carbon atom in a molecule is fundamental to understanding its properties and reactivity. This detailed guide will walk you through the process of identifying and labeling carbon atoms based on their bonding environments, encompassing various geometries like linear, trigonal planar, tetrahedral, and others. We'll explore the VSEPR theory, hybridization, and the impact of different functional groups on carbon atom geometry.
Understanding VSEPR Theory and Hybridization
The Valence Shell Electron Pair Repulsion (VSEPR) theory is the cornerstone of predicting molecular geometry. It posits that electron pairs—both bonding and non-bonding (lone pairs)—repel each other and arrange themselves to minimize this repulsion. This arrangement dictates the overall shape of the molecule.
Hybridization is crucial in understanding the bonding orbitals of carbon. Carbon, with its four valence electrons, can form up to four covalent bonds. Through hybridization, its 2s and 2p orbitals combine to form hybrid orbitals with specific shapes and orientations, influencing the geometry around the carbon atom.
Here's a breakdown of common hybridization types and their corresponding geometries:
sp Hybridization (Linear Geometry)
When a carbon atom forms two sigma bonds and has no lone pairs, it undergoes sp hybridization. The two sp hybrid orbitals are oriented 180° apart, resulting in a linear geometry. This is typically seen in alkynes (molecules containing a triple bond) and some allenes.
Example: In ethyne (acetylene, HC≡CH), both carbon atoms are sp hybridized, resulting in a linear geometry for the entire molecule. Each carbon atom has two sigma bonds and two pi bonds.
sp² Hybridization (Trigonal Planar Geometry)
With three sigma bonds and one lone pair or three sigma bonds and zero lone pairs, carbon undergoes sp² hybridization. The three sp² hybrid orbitals are arranged in a trigonal planar geometry with bond angles of approximately 120°. This is common in alkenes (molecules with a double bond) and carbonyl compounds.
Example: In ethene (ethylene, H₂C=CH₂), each carbon atom is sp² hybridized. The three sigma bonds (two C-H and one C-C) and one pi bond create a planar structure with 120° bond angles around each carbon.
sp³ Hybridization (Tetrahedral Geometry)
When a carbon atom forms four sigma bonds and has no lone pairs, it's sp³ hybridized. The four sp³ hybrid orbitals are arranged in a tetrahedral geometry, with bond angles of approximately 109.5°. This is the most common geometry for carbon atoms in organic molecules, prevalent in alkanes.
Example: In methane (CH₄), the carbon atom is sp³ hybridized. The four C-H bonds are arranged tetrahedrally, with bond angles of approximately 109.5°.
Identifying Carbon Atom Geometry in Complex Molecules
Determining the geometry around carbon atoms becomes more challenging in complex molecules with multiple functional groups. Let's explore some strategies:
Step-by-Step Approach:
-
Identify the Carbon Atom: First, pinpoint the specific carbon atom whose geometry needs to be determined.
-
Count Sigma Bonds and Lone Pairs: Count the number of sigma bonds (single bonds) and lone pairs of electrons connected to the carbon atom. Remember, carbon typically doesn't have lone pairs.
-
Determine Hybridization: Based on the number of sigma bonds, deduce the hybridization:
- Two sigma bonds: sp hybridization (linear geometry)
- Three sigma bonds: sp² hybridization (trigonal planar geometry)
- Four sigma bonds: sp³ hybridization (tetrahedral geometry)
-
Sketch the Geometry: Draw the molecule, focusing on the carbon atom of interest, and arrange the atoms according to the predicted geometry.
-
Consider Steric Effects: In some cases, steric hindrance from bulky substituents can slightly distort bond angles from ideal values.
Examples of Complex Molecules
Let's analyze some examples showcasing diverse geometries:
1. 2-Butene: This molecule has two sp² hybridized carbon atoms (the ones involved in the double bond) and two sp³ hybridized carbon atoms (the methyl groups). The sp² hybridized carbons exhibit trigonal planar geometry, while the sp³ hybridized carbons show tetrahedral geometry.
2. Acetone: The carbonyl carbon in acetone (CH₃COCH₃) is sp² hybridized, exhibiting trigonal planar geometry. The methyl carbons are sp³ hybridized, with tetrahedral geometry.
3. Benzene: Each carbon atom in benzene is sp² hybridized due to one sigma bond with another carbon atom, one sigma bond with a hydrogen, and involvement in a delocalized pi electron system. This results in a planar structure with approximately 120° bond angles.
4. Cyclohexane: In cyclohexane, each carbon atom is sp³ hybridized, exhibiting tetrahedral geometry. However, to minimize steric strain, the molecule adopts a chair conformation, leading to variations in bond angles compared to the ideal tetrahedral angle.
Impact of Functional Groups on Carbon Geometry
Different functional groups can significantly influence the geometry around a carbon atom. For instance:
-
Alcohols (-OH): The carbon atom bonded to the hydroxyl group (-OH) is typically sp³. However, the presence of the oxygen atom can slightly alter bond angles due to its electronegativity.
-
Carboxylic Acids (-COOH): The carbonyl carbon in carboxylic acids is sp², exhibiting trigonal planar geometry, while the carbon adjacent to it is usually sp³.
-
Amines (-NH₂): The carbon atom bonded to the amino group is usually sp³.
-
Ketones and Aldehydes (>C=O): The carbonyl carbon is sp², showing trigonal planar geometry.
-
Ethers (-O-): The carbons bonded to the oxygen atom in ethers are usually sp³.
Understanding the influence of these functional groups is vital for predicting the overall three-dimensional structure of the molecule.
Advanced Considerations: Exceptions and Deviations
While VSEPR theory and hybridization provide excellent predictions, some exceptions and deviations may arise:
-
Steric hindrance: Bulky substituents can cause deviations from ideal bond angles.
-
Ring strain: In small rings, bond angles may deviate significantly from ideal values to accommodate ring closure.
-
Resonance: In molecules with resonance structures, the actual geometry may be an average of the contributing structures.
Applications and Significance
The ability to label carbon atoms with their appropriate geometry is essential in various fields:
-
Organic Chemistry: Predicting reaction mechanisms, reactivity, and stereochemistry often relies on understanding the geometry of carbon atoms.
-
Drug Design: Understanding molecular geometry is crucial for designing drugs that interact effectively with biological targets.
-
Materials Science: The geometry of carbon atoms in materials significantly impacts their physical and chemical properties.
-
Spectroscopy: Interpreting spectroscopic data (NMR, IR) requires a solid understanding of molecular geometry.
Conclusion
Accurately labeling carbon atoms with their appropriate geometry is a fundamental skill in chemistry. By mastering VSEPR theory, hybridization concepts, and the impact of functional groups, you can accurately predict and represent the three-dimensional structures of molecules, enabling a deeper understanding of their properties and behaviors. This comprehensive understanding is essential for success in various scientific disciplines. Remember to always consider potential exceptions and deviations from ideal geometries due to steric factors or other influences. Practice is key to developing this skill, so work through diverse examples and challenge yourself to identify and label the geometry of carbon atoms in complex molecules.
Latest Posts
Latest Posts
-
10 3 Practice Problems Chemistry Answers
Mar 26, 2025
-
1 4 4 Practice Modeling The Rescue Ship Answer Key
Mar 26, 2025
-
Summary Of Contents Of The Dead Mans Pocket
Mar 26, 2025
-
Characters Of Their Eyes Were Watching God
Mar 26, 2025
-
Quotes From The Book And Then There Were None
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Label Each Carbon Atom With The Appropriate Geometry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
