Molar Mass Of Co Nh3 5cl Cl2
Onlines
May 12, 2025 · 5 min read
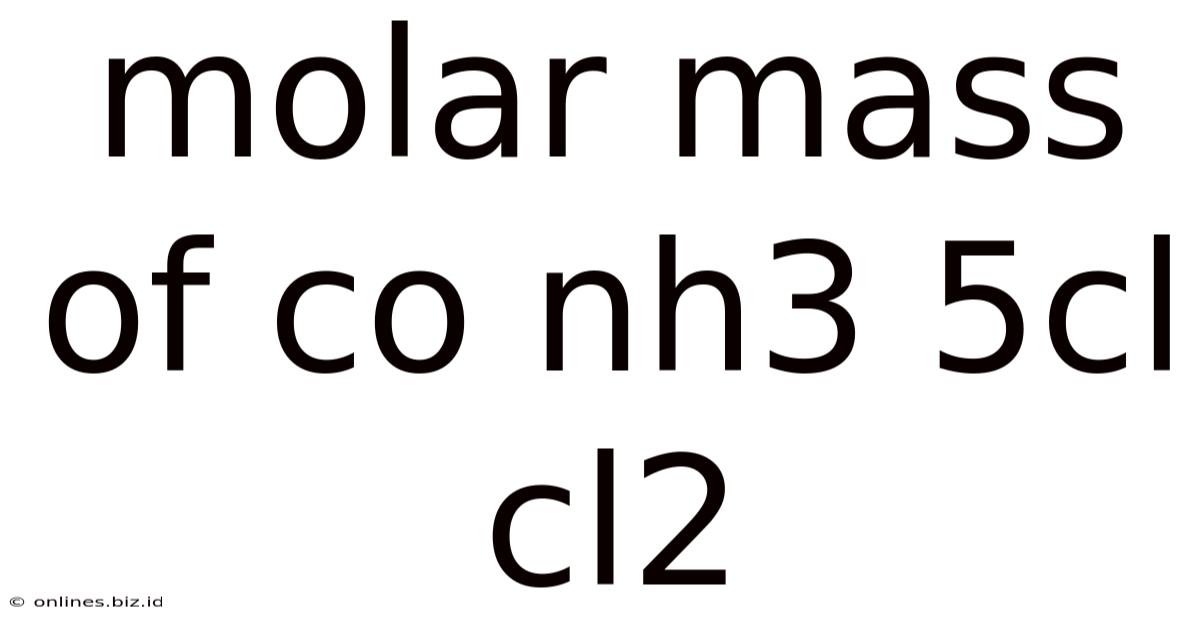
Table of Contents
Calculating the Molar Mass of Co(NH₃)₅ClCl₂
Determining the molar mass of a compound is a fundamental task in chemistry, crucial for various calculations, including stoichiometry, solution preparation, and understanding chemical reactions. This article will guide you through a detailed calculation of the molar mass of the coordination compound, pentaamminechloridocobalt(III) chloride, commonly written as Co(NH₃)₅ClCl₂. We will break down the process step-by-step, explaining the concepts involved and offering insights into the importance of accurate molar mass determination.
Understanding Molar Mass
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). A mole is a fundamental unit in chemistry, representing Avogadro's number (approximately 6.022 x 10²³ particles) of atoms, molecules, ions, or other entities. The molar mass is numerically equal to the atomic weight (or molecular weight) of the substance, but with the units changed from atomic mass units (amu) to grams per mole (g/mol).
Calculating the Molar Mass of Co(NH₃)₅ClCl₂
To calculate the molar mass of Co(NH₃)₅ClCl₂, we need to consider the atomic weights of each element present in the compound and their respective quantities. Let's break down the components:
1. Identifying the Elements and their Atomic Weights
- Cobalt (Co): The atomic weight of cobalt is approximately 58.93 g/mol.
- Nitrogen (N): The atomic weight of nitrogen is approximately 14.01 g/mol.
- Hydrogen (H): The atomic weight of hydrogen is approximately 1.01 g/mol.
- Chlorine (Cl): The atomic weight of chlorine is approximately 35.45 g/mol.
2. Determining the Number of Atoms of Each Element
The chemical formula Co(NH₃)₅ClCl₂ reveals the number of atoms of each element in one molecule of the compound:
- Cobalt (Co): 1 atom
- Nitrogen (N): 5 atoms (from 5 NH₃ ligands)
- Hydrogen (H): 15 atoms (5 NH₃ ligands x 3 H atoms/ligand)
- Chlorine (Cl): 2 atoms (one coordinated and one counterion)
3. Calculating the Total Mass Contribution of Each Element
Now, we multiply the atomic weight of each element by the number of atoms of that element present in the compound:
- Cobalt (Co): 1 atom × 58.93 g/mol = 58.93 g/mol
- Nitrogen (N): 5 atoms × 14.01 g/mol = 70.05 g/mol
- Hydrogen (H): 15 atoms × 1.01 g/mol = 15.15 g/mol
- Chlorine (Cl): 2 atoms × 35.45 g/mol = 70.90 g/mol
4. Summing the Mass Contributions
Finally, we add up the mass contributions of all the elements to obtain the molar mass of Co(NH₃)₅ClCl₂:
58.93 g/mol + 70.05 g/mol + 15.15 g/mol + 70.90 g/mol = 215.03 g/mol
Therefore, the molar mass of Co(NH₃)₅ClCl₂ is approximately 215.03 g/mol.
Significance and Applications of Molar Mass Calculations
Accurate molar mass determination is paramount in numerous chemical applications:
1. Stoichiometric Calculations
Molar mass is fundamental in stoichiometry, allowing us to convert between mass and moles of a substance. This is crucial for balancing chemical equations and predicting the amounts of reactants and products involved in chemical reactions. For instance, knowing the molar mass of Co(NH₃)₅ClCl₂ allows us to calculate the mass needed to prepare a specific molar concentration of a solution.
2. Solution Preparation
When preparing solutions with specific concentrations (e.g., molarity), accurate molar mass is essential. The molarity of a solution is defined as the number of moles of solute per liter of solution. To prepare a solution of a known molarity, you must first calculate the mass of solute required using its molar mass.
3. Determining Empirical and Molecular Formulas
Molar mass plays a vital role in determining the empirical and molecular formulas of unknown compounds. By analyzing the mass percentages of elements in a compound and utilizing its molar mass, we can deduce the relative number of atoms of each element, leading to the determination of both empirical and molecular formulas.
4. Understanding Chemical Reactions
Knowing the molar mass of reactants and products in a chemical reaction allows us to understand the quantitative relationships involved. This understanding is crucial in various fields like industrial chemistry, environmental science, and biochemistry. For example, it's essential to know the molar mass of Co(NH₃)₅ClCl₂ to understand its reactivity in various chemical reactions.
5. Analytical Chemistry
In analytical chemistry, molar mass is used in various techniques, including titration, gravimetric analysis, and spectrophotometry. Accurate molar mass values are crucial for obtaining reliable and accurate results in these analyses.
Potential Sources of Error in Molar Mass Calculations
While the calculation of molar mass is seemingly straightforward, several sources of error can affect the accuracy of the result:
- Impurities in the Compound: If the sample of Co(NH₃)₅ClCl₂ is impure, the calculated molar mass will be affected. Impurities will contribute to the overall mass but not to the actual number of moles of Co(NH₃)₅ClCl₂, leading to an inaccurate result.
- Errors in Atomic Weight Values: The atomic weights used in the calculation are approximate values. Using more precise atomic weights from reliable sources can improve accuracy.
- Rounding Errors: Rounding off atomic weights during the calculation can introduce minor errors, especially when dealing with multiple elements. It's advisable to use as many significant figures as possible throughout the calculation.
- Experimental Errors: If the molar mass is determined experimentally (e.g., through colligative properties), experimental errors in measuring mass, volume, or temperature can affect the accuracy of the result.
Conclusion
Calculating the molar mass of Co(NH₃)₅ClCl₂, or any other compound, is a crucial skill in chemistry. Understanding the process, appreciating the significance of accurate molar mass determination, and being aware of potential sources of error are essential for reliable chemical calculations and analyses. This detailed explanation provides a comprehensive understanding of this fundamental concept, enabling both students and professionals to confidently tackle similar calculations. Remember to always use the most up-to-date and precise values for atomic weights to ensure the highest accuracy in your work.
Latest Posts
Latest Posts
-
According To The Chart When Did A Pdsa Cycle Occur
May 12, 2025
-
Bioflix Activity Gas Exchange The Respiratory System
May 12, 2025
-
Economic Value Creation Is Calculated As
May 12, 2025
-
Which Items Typically Stand Out When You Re Scanning Text
May 12, 2025
-
Assume That Price Is An Integer Variable
May 12, 2025
Related Post
Thank you for visiting our website which covers about Molar Mass Of Co Nh3 5cl Cl2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.