Myofilament With A Knob Like Head
Onlines
Mar 19, 2025 · 6 min read
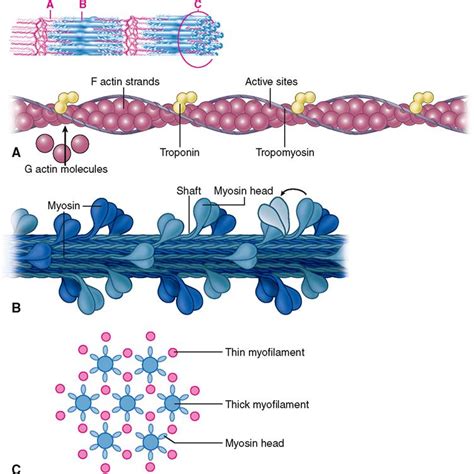
Table of Contents
Myofilaments with Knob-like Heads: A Deep Dive into Myosin and Muscle Contraction
The intricate dance of muscle contraction relies on the precise interaction of numerous proteins. Central to this process are myofilaments, the thread-like structures responsible for generating force. Among these, myosin, with its characteristic knob-like heads, plays a starring role. This article delves deep into the structure, function, and significance of myofilaments featuring these crucial globular heads, providing a comprehensive overview for students, researchers, and anyone fascinated by the wonders of biological mechanics.
The Structure of Myosin: The Molecular Motor
Myosin, a motor protein, is a key component of thick filaments within muscle cells. Its defining feature is the myosin head, also known as the S1 subfragment, a globular structure exhibiting a remarkable ability to bind to and interact with actin, another crucial myofilament. This interaction is the fundamental driving force behind muscle contraction.
Myosin Head Domains: A Closer Look
The myosin head isn't simply a uniform sphere. It's a complex assembly of several domains, each playing a specific role in the contraction cycle. These include:
- Actin-binding domain: This region directly interacts with actin filaments, forming the crucial cross-bridge connection. The precise nature of this interaction is highly specific, ensuring the regulated and controlled movement of myofilaments.
- Nucleotide-binding domain: This domain binds ATP (adenosine triphosphate), the energy source fueling muscle contraction. ATP binding and hydrolysis are central to the conformational changes within the myosin head, driving the power stroke.
- Light chain-binding domain: This region binds to regulatory light chains, influencing the myosin head's activity and modulating the force generation process.
The precise arrangement and interaction of these domains dictate the myosin head's ability to undergo the conformational changes necessary for muscle contraction. The knob-like shape of the head arises from the intricate folding of these domains, optimizing its function within the sarcomere.
The Sarcomere: The Functional Unit of Muscle Contraction
Myofilaments, including actin and myosin, are organized within highly structured units called sarcomeres. The sarcomere is the basic contractile unit of striated muscle (skeletal and cardiac). The arrangement of myosin and actin within the sarcomere contributes significantly to the striated appearance of these muscle types.
Arrangement of Myofilaments within the Sarcomere
Myosin filaments, with their protruding knob-like heads, are located in the center of the sarcomere, forming the A-band. Actin filaments, anchored at the Z-lines, extend from each side, interdigitating with the myosin filaments in the overlapping regions. This precise arrangement allows for the coordinated movement of the myofilaments during contraction.
The Sliding Filament Theory: How Myosin Heads Drive Contraction
The sliding filament theory elegantly explains how muscle contraction occurs. It posits that muscle shortening results from the sliding of actin filaments past myosin filaments, driven by the cyclical interaction of myosin heads with actin.
The Cross-Bridge Cycle: A Detailed Look
The cross-bridge cycle is a series of cyclical steps that describe the interaction between the myosin head and actin filament. These steps are tightly regulated and fueled by ATP:
- ATP Binding: ATP binds to the myosin head, causing its detachment from actin.
- ATP Hydrolysis: ATP is hydrolyzed to ADP and inorganic phosphate (Pi). This causes a conformational change in the myosin head, cocking it into a high-energy state.
- Cross-bridge Formation: The cocked myosin head binds to a new site on the actin filament.
- Power Stroke: The release of ADP and Pi triggers a conformational change in the myosin head, producing a power stroke that pulls the actin filament towards the center of the sarcomere.
- Cross-bridge Detachment: The cycle repeats with the binding of a new ATP molecule.
This cyclical process, occurring simultaneously across numerous myosin heads, generates the force necessary for muscle contraction. The number of active cross-bridges at any given time influences the overall force produced.
Myosin Isozymes: Diverse Functions and Adaptations
Myosin exists in various isoforms, or isozymes, each adapted to specific muscle types and physiological demands. These isoforms differ in their amino acid sequences, impacting their enzymatic activity, contractile properties, and response to regulatory signals.
Variations in Myosin Head Structure
Variations in the amino acid sequence within the myosin head can subtly alter its binding affinity for actin, its ATPase activity, and its overall efficiency in generating force. These differences contribute to the diverse contractile characteristics of different muscle types, from the rapid contractions of skeletal muscles to the sustained contractions of cardiac muscles.
Regulation of Muscle Contraction: Calcium's Crucial Role
Muscle contraction is tightly regulated, ensuring that it occurs only when needed. Calcium ions (Ca2+) play a crucial role in this regulation by controlling the interaction between actin and myosin.
Troponin and Tropomyosin: Key Regulatory Proteins
Tropomyosin, a filamentous protein, wraps around the actin filament, partially blocking the myosin-binding sites. Troponin, a complex of three proteins, binds to tropomyosin and regulates its position. In the absence of Ca2+, tropomyosin blocks myosin binding. When Ca2+ levels rise, it binds to troponin, causing a conformational change that moves tropomyosin, exposing the myosin-binding sites on actin. This initiates the cross-bridge cycle and muscle contraction.
Myofilament Dysfunction and Diseases
Disruptions in the structure or function of myofilaments can lead to various muscle disorders. These can arise from genetic mutations affecting myosin or other myofilament proteins, or from acquired conditions that damage muscle tissue.
Examples of Myofilament-Related Diseases
Several diseases are linked to abnormalities in myosin or other myofilament proteins:
- Familial hypertrophic cardiomyopathy (HCM): A common cause of sudden cardiac death, often associated with mutations in myosin genes.
- Dilated cardiomyopathy (DCM): Characterized by weakened heart muscle, with myosin gene mutations playing a role in some cases.
- Nemaline myopathy: A group of neuromuscular disorders characterized by rod-like structures (nemaline bodies) in muscle fibers. These often involve mutations in proteins interacting with actin or myosin.
Understanding the structure and function of myofilaments, particularly the role of the myosin head, is crucial for diagnosing and treating these and other muscle disorders.
Future Directions and Research
Ongoing research continues to unravel the intricacies of myofilament structure and function. Advances in imaging techniques, such as cryo-electron microscopy, are providing increasingly detailed views of myosin's structure and its interactions with actin. Computational modeling is also playing a crucial role in understanding the dynamics of the cross-bridge cycle and the mechanics of muscle contraction.
Investigating Myosin Isoforms and their Specific Roles
Further research is focused on understanding the specific roles of different myosin isoforms in various muscle types and their contributions to muscle performance and disease. This includes investigating how the subtle variations in myosin head structure affect its function and how these variations might be targeted for therapeutic interventions.
Targeting Myofilaments for Therapeutic Interventions
The myofilament system presents promising therapeutic targets for treating muscle disorders. Understanding the molecular mechanisms underlying myofilament dysfunction paves the way for developing novel therapies aimed at restoring normal muscle function. This might include gene therapy approaches to correct genetic mutations or small molecule drugs to modulate myosin activity.
Conclusion
Myofilaments with their knob-like myosin heads are fundamental to muscle contraction and thus, movement. The intricacies of their structure, the precise mechanisms of their interactions, and the diversity of their isoforms highlight the remarkable elegance and efficiency of biological systems. Continued research into myofilaments will undoubtedly lead to further advancements in our understanding of muscle function, disease, and potential therapeutic interventions. The ongoing investigation into myosin and its crucial knob-like head remains at the forefront of biophysical and biomedical research, promising exciting developments in the future.
Latest Posts
Latest Posts
-
The Following Are All Types Of Friendships Except
Mar 19, 2025
-
Select Tasks That Can Be Completed Directly In The Brain
Mar 19, 2025
-
1 7 Infinite Limits And Limits At Infinity Homework Answer Key
Mar 19, 2025
-
Characterization In The Fall Of The House Of Usher
Mar 19, 2025
-
Themes From Death Of A Salesman
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Myofilament With A Knob Like Head . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
