Negative Ions Have _______________________________ Protons Than Electrons.
Onlines
Mar 14, 2025 · 5 min read
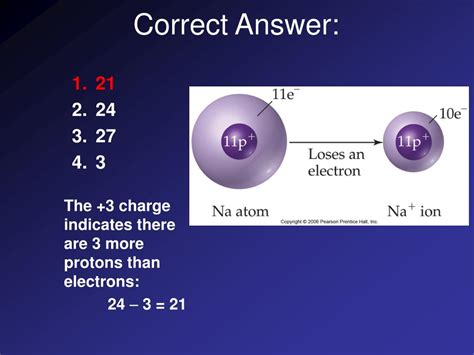
Table of Contents
Negative Ions Have More Electrons Than Protons
Negative ions are atoms or molecules that have gained one or more electrons. This means they possess a net negative charge. The key to understanding this lies in the fundamental principle that negative ions have more electrons than protons. This seemingly simple statement underpins a wide range of scientific phenomena, from atmospheric electricity to the workings of many technologies. Let's delve deeper into this concept, exploring its implications and applications.
Understanding Atomic Structure: Protons, Electrons, and Neutrons
To grasp the concept of negative ions, we need a basic understanding of atomic structure. Atoms are the fundamental building blocks of matter, composed of three primary subatomic particles:
- Protons: Positively charged particles found in the atom's nucleus. The number of protons determines the element's atomic number and its identity on the periodic table.
- Electrons: Negatively charged particles that orbit the nucleus in shells or energy levels. They are significantly lighter than protons and neutrons.
- Neutrons: Neutral particles (no charge) also found in the nucleus. Their presence contributes to an atom's mass but not its charge.
In a neutral atom, the number of protons equals the number of electrons. This balance of positive and negative charges results in a net charge of zero. However, atoms can gain or lose electrons, leading to the formation of ions.
Ionization: The Process of Creating Ions
Ionization is the process by which an atom or molecule gains or loses electrons, acquiring a net electrical charge. This process can occur through various mechanisms, including:
- Friction: Rubbing certain materials together, like walking across a carpet, can transfer electrons, creating static electricity and static cling—often due to the generation of negative ions.
- Radiation: Exposure to high-energy radiation, such as X-rays or gamma rays, can knock electrons out of atoms, forming positive ions (cations).
- Chemical reactions: In many chemical reactions, electrons are transferred between atoms, resulting in the formation of both positive and negative ions. This is fundamental to the formation of ionic compounds.
- Electrical discharges: Lightning strikes are a dramatic example of ionization. The immense electrical energy involved strips electrons from atoms in the air, leading to the formation of ions.
- Radioactive decay: Some radioactive isotopes emit particles that can ionize atoms and molecules.
Negative Ions: An Excess of Electrons
When an atom gains one or more electrons, it becomes negatively charged and is called a negative ion or anion. This is because the number of negatively charged electrons now exceeds the number of positively charged protons. The magnitude of the negative charge depends on the number of extra electrons gained. For instance, an atom that gains one electron has a charge of -1, while an atom that gains two electrons has a charge of -2.
Examples of Negative Ions:
- Chloride ion (Cl⁻): A chlorine atom gains one electron, forming a chloride ion, a common component of table salt (NaCl).
- Oxide ion (O²⁻): An oxygen atom gains two electrons, forming an oxide ion, present in many metal oxides.
- Sulfide ion (S²⁻): A sulfur atom gains two electrons, forming a sulfide ion, found in many minerals.
The Significance of Negative Ions in Various Fields
The presence and behavior of negative ions have significant implications across diverse scientific disciplines:
1. Atmospheric Science and Air Quality:
Negative ions are naturally present in the atmosphere, often associated with cleaner air near waterfalls, forests, and after thunderstorms. These ions are believed by some to have positive effects on human health, though scientific evidence is still being debated and further research is needed. The concentration of negative ions in the air can be affected by various factors, including pollution and atmospheric conditions.
2. Chemistry and Material Science:
Negative ions play a crucial role in chemical bonding, especially in ionic compounds where the electrostatic attraction between positive and negative ions holds the compound together. The properties of ionic compounds, such as their melting points and solubility, are directly related to the strength of these ionic bonds. In material science, understanding the behavior of negative ions is crucial in designing and synthesizing new materials with specific properties.
3. Biology and Medicine:
Although the impact on human health is still under investigation, some studies suggest a potential link between negative ions and improved mood and well-being. However, more robust and controlled studies are needed to confirm these findings.
4. Electronics and Technology:
Negative ions are utilized in various electronic devices and technologies. For example, in some types of vacuum tubes, negative ions are used to enhance conductivity and control the flow of electrons.
Distinguishing Negative Ions from Other Charged Particles
It's crucial to differentiate negative ions from other negatively charged particles:
- Electrons: While electrons are negatively charged, they are fundamental particles, not ions. Ions are formed when atoms gain or lose electrons.
- Negative elementary particles: In particle physics, other negatively charged elementary particles exist, like muons and tau particles. However, these are not ions and are found in different contexts.
The Ongoing Research on Negative Ions
The study of negative ions continues to be an area of active research. Scientists are exploring their roles in:
- Atmospheric chemistry: Understanding the impact of negative ions on air quality and climate change.
- Medical applications: Investigating the potential therapeutic benefits of negative ions.
- Materials science: Developing new materials with enhanced properties through controlled manipulation of negative ions.
- Environmental monitoring: Using negative ion concentration as an indicator of air pollution levels.
Conclusion: The Importance of Understanding Negative Ions
The simple statement that negative ions have more electrons than protons is fundamental to understanding a wide range of scientific phenomena. This excess of electrons is what defines negative ions and makes them integral to many natural processes and technological applications. Ongoing research into negative ions promises to further reveal their diverse roles and applications in the future. Continued investigation will help clarify the sometimes-contradictory information regarding their impact on human health and the environment. While more research is needed to confirm certain claims, the underlying principle of electron excess in negative ions remains a cornerstone of our understanding of chemistry, physics, and the world around us. The exploration of negative ions is a journey into the fascinating world of subatomic particles and their impact on macroscopic systems.
Latest Posts
Latest Posts
-
Pediatric Advanced Life Support Exam A Answers
Mar 14, 2025
-
Chronicle Of A Death Foretold Chapter Summary
Mar 14, 2025
-
Which Of The Following Is True About Conflicts Of Interest
Mar 14, 2025
-
I Could Have Said Many Things But I Said Nothing
Mar 14, 2025
-
What Is The Ram Udimm Vs Sodimm
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Negative Ions Have _______________________________ Protons Than Electrons. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
