Pepsin Can Break A Polypeptide Chain Into ______.
Onlines
Mar 15, 2025 · 5 min read
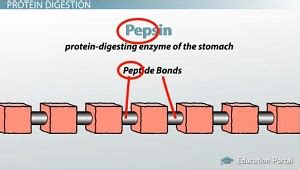
Table of Contents
Pepsin Can Break a Polypeptide Chain into Smaller Polypeptides and Amino Acids
Pepsin, a crucial digestive enzyme, plays a pivotal role in the breakdown of proteins within the stomach. Understanding its function is key to grasping the complexities of human digestion and overall health. This article delves into the specifics of pepsin's action, explaining precisely what it breaks polypeptide chains into, and exploring the broader implications of this process for our bodies.
The Role of Pepsin in Protein Digestion
Our bodies rely on proteins for a multitude of functions, from building and repairing tissues to producing enzymes and hormones. However, proteins ingested through our diet are large, complex molecules that require extensive breakdown before they can be absorbed and utilized by our cells. This is where pepsin comes in.
Pepsin is an endopeptidase, meaning it cleaves peptide bonds within the polypeptide chain, as opposed to exopeptidases, which cleave bonds at the ends. This characteristic is crucial because it allows pepsin to break down large proteins into significantly smaller fragments, setting the stage for further digestion in the small intestine. It's a vital component of the gastric phase of digestion, initiating the process of protein degradation before the partially digested food moves on to the next stage.
The Specificity of Pepsin's Action
Pepsin isn't a random protein-cutter; it exhibits a degree of specificity in its action. It preferentially cleaves peptide bonds involving hydrophobic amino acids, such as phenylalanine, tryptophan, and tyrosine. This selectivity ensures that the breakdown process is not entirely haphazard, but rather follows a pattern that facilitates the eventual absorption of individual amino acids. The exact cleavage sites, however, are influenced by the specific amino acid sequence of the protein being digested, leading to a diverse range of smaller polypeptide chains as products.
Pepsin's Products: Smaller Polypeptides and Amino Acids
So, to answer the question directly: pepsin can break a polypeptide chain into smaller polypeptides and, to a lesser extent, free amino acids. While pepsin's primary action is the generation of smaller polypeptides, some peptide bonds might be cleaved close enough to the end of a chain to release a few free amino acids. However, this is not its main function. The majority of the products are peptides of varying lengths, still requiring further enzymatic digestion in the small intestine.
The Significance of Smaller Polypeptides
The creation of smaller polypeptides is not just a mid-stage process; it’s absolutely crucial for efficient digestion. The smaller size of these peptides allows them to move more easily through the digestive system and interact more effectively with the enzymes in the small intestine. These enzymes, such as trypsin, chymotrypsin, and carboxypeptidases, work in conjunction with pepsin to complete the protein breakdown process. These enzymes are more effective on shorter polypeptide chains, leading to a significantly improved rate of absorption.
Further Digestion in the Small Intestine
The partially digested proteins (smaller polypeptides) moving from the stomach into the duodenum, the first part of the small intestine, trigger the release of more digestive enzymes from the pancreas. These pancreatic enzymes further break down the polypeptides into even smaller fragments, ultimately releasing individual amino acids. These amino acids are then absorbed across the intestinal lining into the bloodstream, ready to be transported to cells throughout the body to perform their essential functions.
The Optimal Environment for Pepsin Activity
Pepsin’s activity is highly dependent on its environment. It is optimally active in the acidic environment of the stomach, maintained by the secretion of hydrochloric acid (HCl) by parietal cells. This acidic pH (approximately 1.5-2.0) is essential for several reasons:
- Enzyme activation: Pepsin is synthesized as an inactive precursor called pepsinogen. The acidic environment of the stomach converts pepsinogen into its active form, pepsin, through a process called autocatalysis, where the already activated pepsin acts on the remaining pepsinogen.
- Protein denaturation: The low pH also denatures proteins, unfolding their three-dimensional structures. This denaturation exposes more peptide bonds, making them more accessible to pepsin's enzymatic action. Without this denaturation, the protein’s tightly folded structure would largely shield many peptide bonds from enzymatic attack.
- Optimal conformation: The acidic environment maintains pepsin in its optimal three-dimensional conformation, allowing it to effectively bind to and cleave peptide bonds. Changes in pH can disrupt this conformation, leading to decreased enzymatic activity.
The Impact of pH Changes on Pepsin Activity
Any significant deviation from the optimal pH range drastically reduces pepsin activity. For example, in conditions like achlorhydria (lack of stomach acid production), pepsin activity is significantly impaired, potentially leading to incomplete protein digestion and nutrient malabsorption. Conversely, conditions that increase stomach acidity can lead to excessive pepsin activity, potentially causing damage to the stomach lining.
Pepsin and Health Implications
The efficient functioning of pepsin is crucial for overall health. Problems with pepsin production or activity can manifest in various ways:
- Protein deficiency: Impaired pepsin function can lead to insufficient protein digestion and absorption, resulting in various nutrient deficiencies. Symptoms can include fatigue, muscle weakness, and impaired immune function.
- Gastrointestinal distress: Excessive pepsin activity, particularly in the absence of sufficient mucosal protection, can damage the stomach lining, leading to gastritis, peptic ulcers, or even stomach cancer.
- Genetic disorders: Rare genetic disorders can affect pepsin production or activity, causing severe digestive problems.
Conclusion: Pepsin's Crucial Role in Digestion
Pepsin's role in breaking down proteins is paramount for nutrient absorption. It initiates the process in the stomach, converting large polypeptide chains into smaller, manageable units, essentially preparing them for the next stage of digestion in the small intestine. Understanding pepsin's function, its optimal environment, and its potential health implications highlights the intricate interplay between enzymes, the digestive system, and overall health. Its ability to break down polypeptide chains into smaller polypeptides and amino acids, a process central to protein digestion and nutrient utilization, emphasizes its indispensable role in human physiology. The specific amino acid sequences of the proteins dictate the precise size and number of the resultant polypeptide chains, underscoring the complexity and efficiency of the digestive process. While the release of free amino acids is a secondary outcome, the primary result is the production of these smaller, more manageable polypeptides, paving the way for their complete breakdown and absorption. Therefore, remembering the importance of this step in the cascade of digestive events is essential for understanding how our bodies utilize proteins efficiently and maintain optimal health.
Latest Posts
Latest Posts
-
Advanced Hardware Lab 6 4 Troubleshoot Monitors And Video
Mar 15, 2025
-
Murder On The Orient Express Chapter Summary
Mar 15, 2025
-
As Added Texture Expands The Form
Mar 15, 2025
-
2 03 Quiz Symbols And Imagery And Mood And Emotion
Mar 15, 2025
-
Empareja Las Palabras De Forma Logica
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Pepsin Can Break A Polypeptide Chain Into ______. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
