Phet Molecular Shapes Vsepr Activity Answer Key
Onlines
Mar 22, 2025 · 6 min read
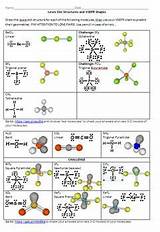
Table of Contents
PHET Molecular Shapes VSEPR Activity: A Comprehensive Guide with Answers
Understanding molecular geometry is crucial in chemistry, impacting a molecule's properties and reactivity. The Valence Shell Electron Pair Repulsion (VSEPR) theory provides a framework for predicting these shapes. This article delves into the PHET Interactive Simulation: Molecular Shapes, offering a comprehensive guide, explanations, and answers to common questions arising from the activity. We'll explore different molecular geometries, lone pair effects, and the application of VSEPR theory.
What is the PHET Molecular Shapes Simulation?
The PhET Interactive Simulations project from the University of Colorado Boulder provides free, engaging educational simulations. Their "Molecular Shapes" simulation allows users to interactively build molecules, explore their 3D structures, and visualize the effects of electron pairs on molecular geometry. This hands-on approach significantly enhances understanding compared to traditional textbook learning.
Key Concepts: VSEPR Theory and Molecular Geometry
Before diving into the simulation, let's recap the fundamental principles:
VSEPR Theory (Valence Shell Electron Pair Repulsion): This theory postulates that electron pairs around a central atom will arrange themselves to minimize repulsion. This arrangement dictates the molecule's overall shape. The key is understanding that both bonding and non-bonding (lone) electron pairs influence the geometry.
Electron Domains: This refers to the regions of space occupied by electrons, including both bonding pairs (electrons shared between atoms) and lone pairs (electrons not involved in bonding).
Molecular Geometry vs. Electron Geometry: These terms are often confused. Electron geometry describes the arrangement of all electron domains (bonding and lone pairs) around the central atom. Molecular geometry, however, describes the arrangement of only the atoms in the molecule, ignoring the lone pairs. Lone pairs influence the bond angles and overall shape but aren't considered when naming the molecular geometry.
Common Molecular Geometries Predicted by VSEPR Theory
The PHET simulation showcases various molecular geometries. Here's a breakdown of the most common ones, along with examples and explanations:
1. Linear (AX₂):
- Electron Geometry: Linear
- Molecular Geometry: Linear
- Bond Angle: 180°
- Example: BeCl₂ (Beryllium chloride) – Two bonding pairs, no lone pairs. The chlorine atoms are positioned 180° apart from each other.
2. Trigonal Planar (AX₃):
- Electron Geometry: Trigonal Planar
- Molecular Geometry: Trigonal Planar
- Bond Angle: 120°
- Example: BF₃ (Boron trifluoride) – Three bonding pairs, no lone pairs. The fluorine atoms are arranged in a flat triangle around the boron atom.
3. Tetrahedral (AX₄):
- Electron Geometry: Tetrahedral
- Molecular Geometry: Tetrahedral
- Bond Angle: 109.5°
- Example: CH₄ (Methane) – Four bonding pairs, no lone pairs. The hydrogen atoms are positioned at the corners of a tetrahedron with carbon at the center.
4. Trigonal Pyramidal (AX₃E):
- Electron Geometry: Tetrahedral
- Molecular Geometry: Trigonal Pyramidal
- Bond Angle: <109.5° (less than due to lone pair repulsion)
- Example: NH₃ (Ammonia) – Three bonding pairs, one lone pair. The lone pair repels the bonding pairs, slightly compressing the bond angles.
5. Bent (AX₂E₂):
- Electron Geometry: Tetrahedral
- Molecular Geometry: Bent
- Bond Angle: <109.5° (significantly less than due to lone pair repulsion)
- Example: H₂O (Water) – Two bonding pairs, two lone pairs. The significant repulsion from the two lone pairs results in a much smaller bond angle.
6. Trigonal Bipyramidal (AX₅):
- Electron Geometry: Trigonal Bipyramidal
- Molecular Geometry: Trigonal Bipyramidal
- Bond Angles: 90°, 120°, 180°
- Example: PCl₅ (Phosphorus pentachloride) – Five bonding pairs, no lone pairs. The molecule has two different bond angles.
7. Octahedral (AX₆):
- Electron Geometry: Octahedral
- Molecular Geometry: Octahedral
- Bond Angle: 90°
- Example: SF₆ (Sulfur hexafluoride) – Six bonding pairs, no lone pairs. The fluorine atoms are located at the corners of an octahedron.
The Influence of Lone Pairs: A Deeper Dive
Lone pairs exert a stronger repulsive force than bonding pairs. This is because lone pairs are closer to the central atom and occupy more space than bonding pairs, which are shared between two nuclei. This leads to distortions in the ideal bond angles. The PHET simulation vividly illustrates this effect by allowing you to visually compare molecules with and without lone pairs.
Working Through the PHET Simulation: Step-by-Step Guide and Answers
The PHET Molecular Shapes simulation is interactive. You build molecules by selecting atoms and bonds. The simulation then automatically displays the 3D structure and provides information about bond angles and molecular geometry.
Here's a suggested workflow:
-
Start with simple molecules: Begin with AX₂ molecules like BeCl₂ to understand the basic linear structure. Gradually increase the number of atoms and lone pairs.
-
Focus on the effect of lone pairs: Compare AX₃ and AX₃E (e.g., BF₃ and NH₃). Observe how the lone pair in ammonia distorts the bond angles. Do the same for AX₂ and AX₂E₂ (e.g., BeCl₂ and H₂O).
-
Explore larger molecules: Experiment with AX₅ and AX₆ structures like PCl₅ and SF₆ to visualize the more complex geometries. Note the different bond angles present.
-
Use the simulation's tools: The simulation provides tools to rotate the molecules, zoom in/out, and view different representations (ball-and-stick, space-filling). Utilize these features to fully grasp the three-dimensional nature of these structures.
-
Predict shapes before building: Challenge yourself by predicting the molecular geometry before building the molecule in the simulation. This strengthens your understanding of VSEPR theory.
Example Problem and Solution:
Problem: Predict the electron geometry and molecular geometry of SF₄ (Sulfur tetrafluoride).
Solution:
- Step 1: Count valence electrons: Sulfur has 6 valence electrons, and each fluorine has 7, totaling 34 valence electrons.
- Step 2: Draw the Lewis structure: This will show sulfur as the central atom with four single bonds to fluorine atoms and one lone pair on sulfur.
- Step 3: Determine electron domains: There are five electron domains (four bonding pairs and one lone pair).
- Step 4: Determine electron geometry: With five electron domains, the electron geometry is trigonal bipyramidal.
- Step 5: Determine molecular geometry: Due to the lone pair's presence, the molecular geometry is seesaw (or distorted tetrahedral). The lone pair occupies an equatorial position to minimize repulsion. The PHET simulation will confirm this.
Advanced Applications and Extensions
The PHET simulation provides a strong foundation for understanding VSEPR theory. However, more complex scenarios require deeper analysis:
- Multiple Central Atoms: Molecules with more than one central atom require considering the geometry around each central atom individually.
- Resonance Structures: Molecules with resonance structures may exhibit variations in bond lengths and angles that deviate slightly from VSEPR predictions.
- Hybridization: The concept of orbital hybridization (sp, sp², sp³, etc.) provides a more detailed explanation of the bonding in these molecules. The PHET simulation doesn't directly address hybridization, but understanding it complements VSEPR theory.
- Polarity: Molecular geometry is crucial in determining molecular polarity. The simulation helps visualize the molecule's shape, allowing you to predict the overall dipole moment based on individual bond dipoles.
Conclusion: Mastering Molecular Shapes with the PHET Simulation
The PHET Molecular Shapes simulation offers an invaluable tool for learning and understanding VSEPR theory. Its interactive nature allows users to actively explore molecular geometries, visualize the effects of lone pairs, and solidify their understanding of this crucial chemical concept. By working through the simulation and applying the principles outlined in this guide, you'll gain a much deeper and more intuitive grasp of molecular shapes and their implications. Remember to practice, experiment with different molecules, and use the simulation's features to fully explore the world of molecular geometry. Remember that consistent practice is key to mastering this concept. Through this hands-on approach coupled with theoretical understanding, you will be well-equipped to tackle more complex chemical concepts.
Latest Posts
Latest Posts
-
Risk Or Reward Chapter 7 Lesson 6
Mar 23, 2025
-
When You Evaluate An Online Document For Sponsorship You Should
Mar 23, 2025
-
Amoeba Sisters Video Recap Multiple Alleles Blood Types
Mar 23, 2025
-
I Accept The Point That Whenever Learning Occurs
Mar 23, 2025
-
Suppose The Canadian Government Has Decided
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Phet Molecular Shapes Vsepr Activity Answer Key . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
