Please Predict The Products For Each Of The Following Reactions
Onlines
Mar 18, 2025 · 6 min read
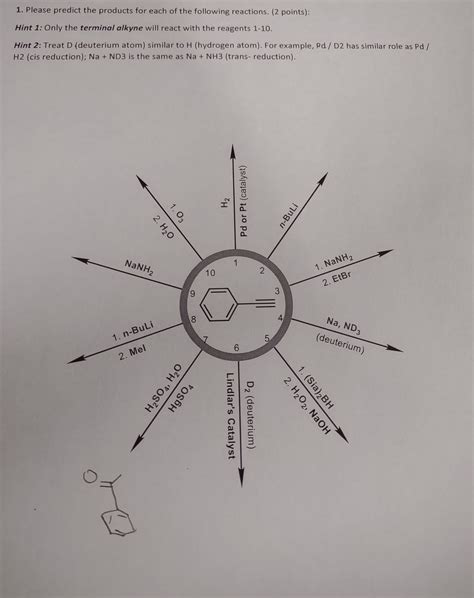
Table of Contents
Predicting Chemical Reaction Products: A Comprehensive Guide
Predicting the products of chemical reactions is a fundamental skill in chemistry. It requires a solid understanding of chemical principles, including reactivity series, reaction types (acid-base, redox, precipitation, etc.), and stoichiometry. This article will explore various reaction types and provide a systematic approach to predicting products, offering examples and explanations along the way. We'll delve into both simple and more complex reactions, illustrating the reasoning behind each prediction.
Understanding Reaction Types: The Key to Prediction
Before we dive into specific examples, let's review the major types of chemical reactions:
1. Synthesis (Combination) Reactions
In synthesis reactions, two or more substances combine to form a single, more complex product. The general form is: A + B → AB.
Example: The reaction between sodium (Na) and chlorine (Cl₂) to form sodium chloride (NaCl):
2Na(s) + Cl₂(g) → 2NaCl(s)
Prediction Strategy: Identify the reactants and consider their combining capacity (valency). The product will be a compound formed by the combination of these reactants.
2. Decomposition Reactions
Decomposition reactions involve the breakdown of a single compound into two or more simpler substances. The general form is: AB → A + B. These reactions often require energy input, such as heat or electricity.
Example: The decomposition of calcium carbonate (CaCO₃) into calcium oxide (CaO) and carbon dioxide (CO₂):
CaCO₃(s) → CaO(s) + CO₂(g)
Prediction Strategy: Consider the stability of the reactant. Unstable compounds tend to decompose into more stable products. Look for patterns like the formation of gases (like CO₂, O₂, or H₂).
3. Single Displacement (Substitution) Reactions
In single displacement reactions, a more reactive element replaces a less reactive element in a compound. The general form is: A + BC → AC + B. The reactivity is determined by activity series (e.g., for metals).
Example: The reaction between zinc (Zn) and hydrochloric acid (HCl):
Zn(s) + 2HCl(aq) → ZnCl₂(aq) + H₂(g)
Prediction Strategy: Refer to an activity series. A more reactive element (higher on the series) will displace a less reactive element from its compound.
4. Double Displacement (Metathesis) Reactions
Double displacement reactions involve the exchange of ions between two compounds. The general form is: AB + CD → AD + CB. These often lead to the formation of a precipitate, a gas, or water.
Example: The reaction between silver nitrate (AgNO₃) and sodium chloride (NaCl):
AgNO₃(aq) + NaCl(aq) → AgCl(s) + NaNO₃(aq)
Prediction Strategy: Consider the solubility rules to predict the formation of a precipitate. Look for the formation of a weak electrolyte (like water) or a gas.
5. Combustion Reactions
Combustion reactions involve the rapid reaction of a substance with oxygen, usually producing heat and light. Complete combustion of hydrocarbons produces carbon dioxide and water.
Example: The combustion of methane (CH₄):
CH₄(g) + 2O₂(g) → CO₂(g) + 2H₂O(g)
Prediction Strategy: For hydrocarbons, the products are typically CO₂ and H₂O in complete combustion. Incomplete combustion can produce carbon monoxide (CO) or carbon (C) as well.
6. Redox (Oxidation-Reduction) Reactions
Redox reactions involve the transfer of electrons between reactants. One substance is oxidized (loses electrons), and another is reduced (gains electrons). These reactions often involve changes in oxidation states.
Example: The reaction between iron (Fe) and oxygen (O₂):
4Fe(s) + 3O₂(g) → 2Fe₂O₃(s)
Prediction Strategy: Determine the oxidation states of the reactants and predict the changes in oxidation states during the reaction. Use balancing techniques to ensure the number of electrons lost equals the number of electrons gained.
Predicting Products: Step-by-Step Approach
Let's apply these principles to predict the products of various reactions:
Example 1: Reaction between potassium (K) and water (H₂O)
Potassium is a highly reactive alkali metal. It reacts vigorously with water, producing a metal hydroxide and hydrogen gas.
Prediction:
2K(s) + 2H₂O(l) → 2KOH(aq) + H₂(g)
Reasoning: This is a single displacement reaction where potassium displaces hydrogen from water. Potassium hydroxide is formed, and hydrogen gas is released.
Example 2: Reaction between sulfuric acid (H₂SO₄) and sodium hydroxide (NaOH)
This is a classic acid-base neutralization reaction. The products will be a salt and water.
Prediction:
H₂SO₄(aq) + 2NaOH(aq) → Na₂SO₄(aq) + 2H₂O(l)
Reasoning: The hydrogen ions (H⁺) from sulfuric acid react with the hydroxide ions (OH⁻) from sodium hydroxide to form water. The remaining ions (Na⁺ and SO₄²⁻) combine to form sodium sulfate.
Example 3: Reaction between calcium chloride (CaCl₂) and silver nitrate (AgNO₃)
This is a double displacement reaction that leads to the formation of a precipitate.
Prediction:
CaCl₂(aq) + 2AgNO₃(aq) → Ca(NO₃)₂(aq) + 2AgCl(s)
Reasoning: Silver chloride (AgCl) is insoluble in water and precipitates out of the solution. Calcium nitrate (Ca(NO₃)₂) remains dissolved.
Example 4: Combustion of propane (C₃H₈)
Propane is a hydrocarbon. Complete combustion in oxygen produces carbon dioxide and water.
Prediction:
C₃H₈(g) + 5O₂(g) → 3CO₂(g) + 4H₂O(g)
Reasoning: Balance the equation to ensure the number of atoms of each element is equal on both sides.
Example 5: Reaction between iron(III) oxide (Fe₂O₃) and carbon monoxide (CO)
This is a redox reaction where iron(III) oxide is reduced, and carbon monoxide is oxidized.
Prediction:
Fe₂O₃(s) + 3CO(g) → 2Fe(s) + 3CO₂(g)
Reasoning: Iron(III) in Fe₂O₃ is reduced to elemental iron (Fe), while carbon in CO is oxidized to carbon dioxide (CO₂).
Advanced Considerations and Complex Reactions
Predicting products becomes more challenging with complex reactions involving multiple steps or organic compounds. Factors like reaction conditions (temperature, pressure, presence of catalysts) significantly influence the outcome.
Organic Reactions: Organic chemistry involves a vast array of reactions with diverse mechanisms. Predicting products requires a strong understanding of functional groups, reaction mechanisms (e.g., SN1, SN2, E1, E2), and reaction kinetics.
Equilibria: Many reactions are reversible, reaching a state of equilibrium where the rates of the forward and reverse reactions are equal. Predicting the product distribution requires knowledge of equilibrium constants (K) and Le Chatelier's principle.
Catalysis: Catalysts can dramatically alter reaction pathways, leading to different products compared to uncatalyzed reactions. The presence of a catalyst must be considered when predicting products.
Conclusion
Predicting the products of chemical reactions is a crucial skill in chemistry. A systematic approach, starting with identifying the reaction type and considering factors like reactivity series, solubility rules, and oxidation states, is essential. While simple reactions can be predicted relatively easily, understanding reaction mechanisms and considering factors such as reaction conditions, equilibria, and catalysis becomes increasingly important for more complex reactions, especially in organic chemistry. Practice and a solid foundation in chemical principles are key to mastering this skill. Remember to always balance chemical equations to accurately reflect the stoichiometry of the reaction.
Latest Posts
Latest Posts
-
5 2 Warm Up Learning Resource Knowledge Quiz
Mar 18, 2025
-
Estimate The Number Of Heartbeats In A Pound Of Butter
Mar 18, 2025
-
Art Labeling Activity Summary Of Epithelial Tissues
Mar 18, 2025
-
The Martian Chronicles The Martian Summary
Mar 18, 2025
-
Examples Of Questions That Focus On Process Include
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Please Predict The Products For Each Of The Following Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
