Practice Isotope Calculations #2 Answer Key
Onlines
Mar 13, 2025 · 7 min read
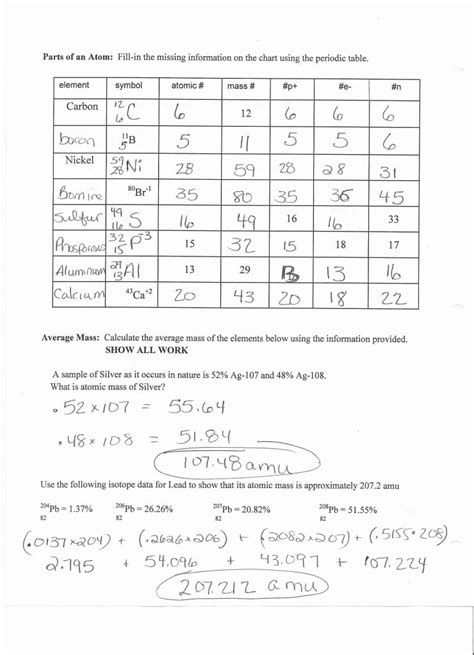
Table of Contents
Practice Isotope Calculations #2: Answer Key and Detailed Explanations
This comprehensive guide provides detailed solutions and explanations for a second set of practice problems focused on isotope calculations. Mastering these calculations is crucial for understanding various fields, including nuclear chemistry, geochemistry, and environmental science. We'll cover a range of problem types, from basic isotopic abundance calculations to more complex scenarios involving radioactive decay. Each problem will be meticulously solved, emphasizing the underlying principles and methodologies.
Understanding Isotopes and Isotopic Abundance
Before diving into the answer key, let's briefly review the fundamental concepts. Isotopes are atoms of the same element that have the same number of protons but different numbers of neutrons. This difference in neutron number results in variations in atomic mass. Isotopic abundance refers to the relative proportion of each isotope of an element present in a naturally occurring sample. These abundances are typically expressed as percentages.
Key Formulae:
-
Average Atomic Mass: Average atomic mass = Σ (mass of isotope * fractional abundance)
-
Radioactive Decay: The rate of radioactive decay follows first-order kinetics and is governed by the equation: N<sub>t</sub> = N<sub>0</sub>e<sup>-λt</sup>, where:
- N<sub>t</sub> is the amount of the isotope remaining after time t
- N<sub>0</sub> is the initial amount of the isotope
- λ is the decay constant
- t is the time elapsed
Practice Problems and Solutions: Answer Key
This section presents the solutions and detailed explanations for a series of practice problems on isotope calculations.
Problem 1: Calculating Average Atomic Mass
-
Problem: Element X exists as two isotopes: <sup>63</sup>X (62.93 amu, 69.17% abundance) and <sup>65</sup>X (64.93 amu, 30.83% abundance). Calculate the average atomic mass of element X.
-
Solution:
Average atomic mass = (62.93 amu * 0.6917) + (64.93 amu * 0.3083) = 43.53 amu + 20.01 amu = 63.54 amu
-
Explanation: We apply the formula for average atomic mass, weighting each isotope's mass by its fractional abundance. The result, 63.54 amu, represents the weighted average mass of element X as found in nature.
Problem 2: Determining Isotopic Abundance
-
Problem: Element Y has an average atomic mass of 107.87 amu. It exists as two isotopes: <sup>107</sup>Y (106.91 amu) and <sup>109</sup>Y (108.91 amu). Determine the percent abundance of each isotope.
-
Solution:
Let x be the fractional abundance of <sup>107</sup>Y. Then (1-x) is the fractional abundance of <sup>109</sup>Y.
107.87 amu = (106.91 amu * x) + (108.91 amu * (1-x))
Solving for x:
107.87 = 106.91x + 108.91 - 108.91x -1.04 = -2x x = 0.52
Therefore, the abundance of <sup>107</sup>Y is 52% and the abundance of <sup>109</sup>Y is 48%.
-
Explanation: We set up an equation using the average atomic mass and the known masses and abundances. Solving this equation allows us to determine the fractional abundance of each isotope, which we then convert to percentages.
Problem 3: Radioactive Decay – Half-Life Calculation
-
Problem: A sample of <sup>14</sup>C initially contains 100 grams. The half-life of <sup>14</sup>C is 5730 years. How many grams of <sup>14</sup>C remain after 11460 years?
-
Solution:
After one half-life (5730 years), 50 grams remain. After two half-lives (11460 years), 25 grams remain.
-
Explanation: This problem uses the concept of half-life. Each half-life reduces the amount of the radioactive isotope by half. After 11460 years (two half-lives), only a quarter of the initial amount remains.
Problem 4: Radioactive Decay – Using the Decay Equation
-
Problem: A sample of <sup>238</sup>U decays with a decay constant (λ) of 1.54 x 10<sup>-10</sup> per year. If the initial amount (N<sub>0</sub>) is 1000 grams, how much remains after 1 billion years (t)?
-
Solution:
We use the radioactive decay equation: N<sub>t</sub> = N<sub>0</sub>e<sup>-λt</sup>
N<sub>t</sub> = 1000g * e<sup>-(1.54 x 10<sup>-10</sup> year<sup>-1</sup> * 1 x 10<sup>9</sup> year)</sup> N<sub>t</sub> ≈ 1000g * e<sup>-0.154</sup> N<sub>t</sub> ≈ 857 grams
-
Explanation: This problem utilizes the exponential decay equation to calculate the remaining amount of <sup>238</sup>U after a specified time. The decay constant and the initial amount are used as inputs.
Problem 5: Isotope Dilution
-
Problem: A 10.0 gram sample of a mixture containing an unknown amount of <sup>15</sup>N is enriched with 1.0 gram of <sup>15</sup>N tracer (99% <sup>15</sup>N). After mixing and appropriate separation, the final <sup>15</sup>N abundance in the sample is found to be 50%. Calculate the initial amount of <sup>15</sup>N in the unknown sample.
-
Solution:
The added tracer contains 0.99g of <sup>15</sup>N (99% of 1g). The total amount of <sup>15</sup>N after mixing is 10.0g total sample * 0.50 = 5.0g.
Initial amount of <sup>15</sup>N = 5.0g (Total <sup>15</sup>N after mixing) - 0.99g (Added tracer) = 4.01g
-
Explanation: This problem showcases the principle of isotope dilution, commonly used in analytical chemistry. Adding a known amount of a labeled isotope helps determine the original amount of the isotope in the mixture.
Problem 6: Mass Spectrometry and Isotopic Ratios
-
Problem: A mass spectrometer analysis of a water sample reveals the following isotopic ratios: <sup>16</sup>O/<sup>18</sup>O = 450 and <sup>1</sup>H/<sup>2</sup>H = 6400. Explain what this data indicates.
-
Solution: These ratios represent the relative abundance of different isotopes of oxygen and hydrogen in the water sample. A high <sup>16</sup>O/<sup>18</sup>O ratio indicates a predominance of the lighter oxygen isotope, while a high <sup>1</sup>H/<sup>2</sup>H ratio shows a greater abundance of protium (<sup>1</sup>H) compared to deuterium (<sup>2</sup>H). These ratios can be used to infer information about the water source, its history, and possible environmental influences.
-
Explanation: Mass spectrometry allows the precise measurement of isotope ratios. These ratios are invaluable tools in various fields, including environmental science and paleoclimatology, to understand different processes and systems.
Advanced Isotope Calculations and Applications
The previous problems illustrate fundamental isotope calculations. However, several more advanced techniques and applications deserve further exploration:
Radiocarbon Dating (<sup>14</sup>C Dating)
Radiocarbon dating is a cornerstone technique in archaeology and geology, used to determine the age of organic materials up to around 50,000 years old. It relies on the decay of <sup>14</sup>C, a radioactive isotope of carbon. The method involves measuring the remaining <sup>14</sup>C in a sample and comparing it to the known initial abundance, enabling the estimation of the time elapsed since the organism died. The half-life of <sup>14</sup>C is used in this calculation.
Stable Isotope Analysis
Stable isotope analysis investigates the ratios of non-radioactive isotopes. These ratios are often subtly altered by environmental processes, providing insights into various parameters like climate, diet, and migration patterns. Elements frequently studied using stable isotope analysis include carbon (<sup>13</sup>C/<sup>12</sup>C), nitrogen (<sup>15</sup>N/<sup>14</sup>N), oxygen (<sup>18</sup>O/<sup>16</sup>O), and hydrogen (<sup>2</sup>H/<sup>1</sup>H).
Isotope Geochemistry
Isotope geochemistry uses isotopic ratios to study geological processes and Earth's evolution. The isotopic composition of rocks and minerals can provide information on their formation age, the source materials involved, and temperature conditions. This area of study uses a variety of isotopic systems, including those involving uranium, lead, strontium, and neodymium.
Medical Applications of Isotopes
Radioactive isotopes have significant applications in medicine, primarily in diagnostics and treatment. Radioactive tracers, such as iodine-131 and technetium-99m, are used in medical imaging to visualize organs and tissues. Radiotherapy employs isotopes like cobalt-60 and iridium-192 to deliver targeted radiation to cancerous cells.
Conclusion
Mastering isotope calculations is vital for understanding numerous scientific disciplines. This guide has provided a thorough examination of several problem types, including average atomic mass calculations, isotopic abundance determination, radioactive decay problems, isotope dilution calculations, and applications such as radiocarbon dating and mass spectrometry. Remember that these calculations are not just theoretical exercises; they represent essential tools for investigating a wide range of natural and applied scientific phenomena. By continually practicing these calculations and exploring more advanced applications, you'll build a strong foundation in this crucial area of science.
Latest Posts
Latest Posts
-
Official Advancement Handbooks Are Available From What Official Source
Mar 14, 2025
-
Knowledge Drill 9 7 Serum Appearance
Mar 14, 2025
-
Unit 8 Worksheet 1 Mole Relationships
Mar 14, 2025
-
Lesson 2 Eggzactly M1 67 Answer Key
Mar 14, 2025
-
Separation Of The Components Of A Mixture Pre Lab Answers
Mar 14, 2025
Related Post
Thank you for visiting our website which covers about Practice Isotope Calculations #2 Answer Key . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
