Provide The Correct Iupac Name For The Compound Shown Here.
Onlines
Mar 05, 2025 · 7 min read
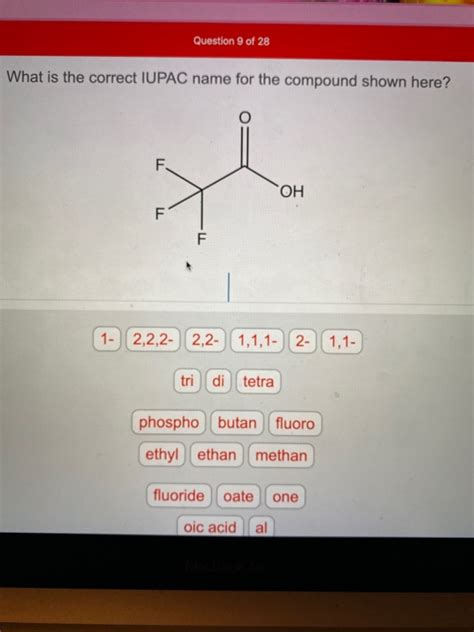
Table of Contents
Providing the Correct IUPAC Name for Organic Compounds: A Comprehensive Guide
Naming organic compounds might seem daunting at first, but with a systematic approach, it becomes manageable. The International Union of Pure and Applied Chemistry (IUPAC) provides a set of rules to ensure a unique and unambiguous name for every organic molecule. This comprehensive guide will walk you through the process, equipping you with the knowledge to confidently name various organic compounds. We'll cover alkanes, alkenes, alkynes, alcohols, aldehydes, ketones, carboxylic acids, and more, providing examples and tackling potential complexities.
Understanding IUPAC Nomenclature: The Foundation
The IUPAC system is built upon a series of rules prioritizing the longest carbon chain, identifying functional groups, and assigning locants (numbers) to indicate the position of substituents. Understanding these core principles is paramount to accurately naming any organic compound.
1. Identifying the Parent Chain: The Backbone of the Molecule
The parent chain is the longest continuous chain of carbon atoms in the molecule. This forms the base name of the compound. For example, in a branched alkane, even if there are chains branching off, you must locate the longest continuous chain to determine the parent alkane's name.
- Example: Consider a molecule with a 5-carbon chain and a 2-carbon branch. The parent chain is the 5-carbon chain, making the parent alkane pentane.
2. Numbering the Carbon Chain: Assigning Locants
Once the parent chain is identified, each carbon atom is numbered sequentially. Numbering starts from the end that gives the substituents the lowest possible numbers. The goal is to minimize the numbers used in the name. If multiple substituents are present, prioritize the lowest numbers for the substituents' positions.
- Example: If a substituent is on the second carbon and another is on the fourth carbon, we number from the end closer to the substituent at the lower number (meaning we'd number the carbons 1-5 rather than 5-1).
3. Identifying and Naming Substituents: Branches and Functional Groups
Substituents are atoms or groups of atoms attached to the parent chain. They are named according to their structure and added as prefixes to the parent chain's name. The positions of these substituents are indicated by their locants (numbers).
- Alkyl Groups: These are substituents derived from alkanes by removing a hydrogen atom (e.g., methyl -CH₃, ethyl -CH₂CH₃, propyl -CH₂CH₂CH₃).
- Functional Groups: These are specific groups of atoms within a molecule that determine the molecule’s chemical properties and influence its reactivity (e.g., hydroxyl -OH for alcohols, carboxyl -COOH for carboxylic acids).
4. Combining the Information: Constructing the IUPAC Name
The complete IUPAC name is constructed by combining the following:
- Locants: Numbers indicating the positions of substituents on the parent chain.
- Substituent Names: Names of the substituents attached to the parent chain.
- Parent Chain Name: Name of the longest continuous carbon chain.
- Example: A compound with a 5-carbon chain (pentane) and a methyl group on the second carbon would be named 2-methylpentane. If there are two methyl groups on the second carbon, it would be 2,2-dimethylpentane.
IUPAC Nomenclature for Specific Compound Classes
Let's delve into the specifics of naming various compound classes.
1. Alkanes: The Simplest Hydrocarbons
Alkanes are saturated hydrocarbons (containing only single bonds) with the general formula C<sub>n</sub>H<sub>2n+2</sub>. Their names follow a straightforward system:
- Methane (1 carbon)
- Ethane (2 carbons)
- Propane (3 carbons)
- Butane (4 carbons)
- Pentane (5 carbons)
- Hexane (6 carbons)
- Heptane (7 carbons)
- Octane (8 carbons)
- Nonane (9 carbons)
- Decane (10 carbons)
Branched alkanes follow the rules outlined earlier: identify the longest continuous chain, number the carbons, name and locate the substituents.
2. Alkenes and Alkynes: Unsaturated Hydrocarbons
Alkenes contain at least one carbon-carbon double bond (C=C), while alkynes have at least one carbon-carbon triple bond (C≡C).
-
Alkenes: The suffix "-ene" is used. The position of the double bond is indicated by the lower number of the two carbons involved in the double bond.
-
Alkynes: The suffix "-yne" is used. The position of the triple bond is indicated by the lower number of the two carbons involved in the triple bond.
-
Example: CH₂=CHCH₂CH₃ is named but-1-ene (the double bond is between carbons 1 and 2). CH≡CCH₂CH₃ is named but-1-yne.
3. Alcohols: Containing the Hydroxyl Group
Alcohols contain the hydroxyl group (-OH). The suffix "-ol" is used, and the position of the hydroxyl group is indicated by a number.
- Example: CH₃CH₂CH₂OH is named propan-1-ol. CH₃CH(OH)CH₃ is named propan-2-ol.
4. Aldehydes and Ketones: Carbonyl Compounds
Aldehydes and ketones contain a carbonyl group (C=O).
-
Aldehydes: The carbonyl group is at the end of the carbon chain. The suffix "-al" is used.
-
Ketones: The carbonyl group is within the carbon chain. The suffix "-one" is used, and the position of the carbonyl group is indicated by a number.
-
Example: CH₃CHO is named ethanal. CH₃COCH₃ is named propan-2-one (commonly known as acetone).
5. Carboxylic Acids: Containing the Carboxyl Group
Carboxylic acids contain the carboxyl group (-COOH). The suffix "-oic acid" is used. The carbon of the carboxyl group is always carbon number 1.
- Example: CH₃COOH is named ethanoic acid (commonly known as acetic acid).
6. Amines: Containing the Amino Group
Amines contain the amino group (-NH₂). The prefix "amino-" is used, and the position of the amino group is indicated by a number.
- Example: CH₃CH(NH₂)CH₃ is named 2-aminopropane.
7. Halogenated Compounds: Containing Halogens
Halogens (fluorine, chlorine, bromine, iodine) are named as fluoro-, chloro-, bromo-, or iodo-. Their positions are indicated by numbers.
- Example: CH₃CHClCH₃ is named 2-chloropropane.
Handling Complex Structures: Prioritizing and Applying the Rules
When dealing with complex molecules containing multiple substituents and functional groups, a hierarchy of rules is crucial. The IUPAC system prioritizes functional groups, often determining the suffix and base name. Here’s a simplified prioritization:
- Carboxylic acids
- Anhydrides
- Esters
- Amides
- Nitriles
- Aldehydes
- Ketones
- Alcohols
- Amines
- Alkenes/Alkynes
- Haloalkanes
For instance, a molecule with both an alcohol and a ketone group will be named as a ketone (higher priority), with the alcohol group treated as a substituent (hydroxy-). Careful attention to numbering and prioritizing substituents is essential to generating the correct IUPAC name.
Navigating Ambiguities and Exceptions: A Note on Complexity
While the IUPAC system strives for uniformity, complexities arise with intricate molecular structures. Isomers (molecules with the same formula but different arrangements) require precise locants and prefixes to distinguish them. Cyclic compounds introduce additional naming conventions, requiring the identification of the ring system and the numbering of the ring carbons. Understanding stereochemistry (spatial arrangement of atoms) may also be necessary for a complete and accurate name, especially when dealing with chiral centers. Consult more advanced resources if you encounter such challenges.
Conclusion: Mastering the Art of IUPAC Nomenclature
Mastering IUPAC nomenclature involves consistent application of rules, a keen eye for detail, and a systematic approach. While the process might initially appear complex, understanding the fundamental principles of identifying the parent chain, numbering, naming substituents, and prioritizing functional groups allows for the accurate naming of a wide array of organic compounds. By practicing with various examples and referring to comprehensive resources, you can build confidence and proficiency in this essential aspect of organic chemistry. This detailed guide serves as a foundation, encouraging further exploration of the intricacies of IUPAC naming conventions as you tackle more challenging structures. Remember, practice is key to mastering this vital skill.
Latest Posts
Latest Posts
-
We Have Always Lived In A Castle Sparknotes
Mar 05, 2025
-
El Capitan Era El Mas Feliz Correct Incorrect
Mar 05, 2025
-
Which Of These Statements Accurately Describes A Dts Role
Mar 05, 2025
-
Julius Caesar Act 2 Character Map
Mar 05, 2025
-
How Many Chapters Are In Divergent
Mar 05, 2025
Related Post
Thank you for visiting our website which covers about Provide The Correct Iupac Name For The Compound Shown Here. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
