Question Volkswagen Draw The Major Sn2
Onlines
Mar 03, 2025 · 5 min read
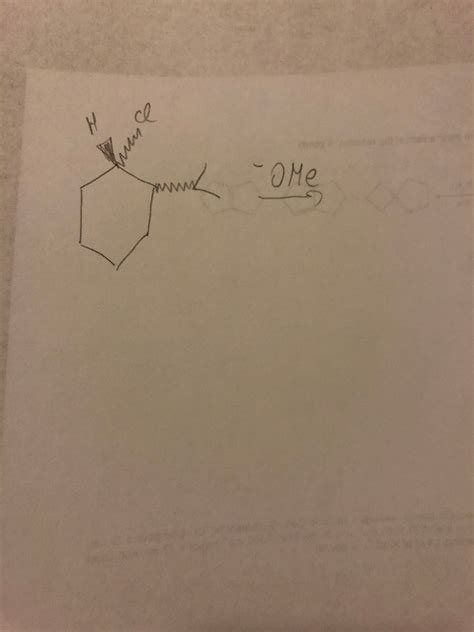
Table of Contents
Decoding the Volkswagen SN2 Reaction: A Deep Dive into Nucleophilic Substitution
The Volkswagen SN2 reaction, while not an officially named reaction in organic chemistry textbooks, is a useful mnemonic device to understand and remember the crucial aspects of the SN2 (Substitution Nucleophilic Bimolecular) reaction mechanism. This article delves into the intricacies of the SN2 reaction, using the "Volkswagen" analogy to aid comprehension, and explores its key features, factors influencing its rate, and its application in organic synthesis.
Understanding the SN2 Reaction: A Nucleophilic Attack
The SN2 reaction is a fundamental concept in organic chemistry. It's a type of substitution reaction where a nucleophile (a species with a lone pair of electrons) attacks an electrophilic carbon atom, leading to the displacement of a leaving group. The term "bimolecular" signifies that the rate-determining step involves two molecules: the nucleophile and the substrate.
Key Characteristics of the SN2 Reaction:
- Concerted Mechanism: The nucleophile attacks the substrate simultaneously as the leaving group departs. This occurs in a single, concerted step, without the formation of any intermediate carbocations.
- Stereochemistry: The SN2 reaction leads to inversion of configuration at the stereocenter. This means that if the starting material is chiral, the product will have the opposite stereochemistry. This is often referred to as a Walden inversion.
- Substrate Dependence: The reaction is favored by primary (methyl or 1°) alkyl halides. Secondary (2°) alkyl halides can undergo SN2 reactions, but the rate is significantly slower. Tertiary (3°) alkyl halides generally do not participate in SN2 reactions because of steric hindrance.
- Nucleophile Dependence: The reaction rate is significantly influenced by the strength of the nucleophile. Stronger nucleophiles react faster. Good nucleophiles often possess a negative charge or a lone pair of electrons on an electronegative atom.
- Leaving Group Dependence: The leaving group's ability to stabilize the negative charge after departure is crucial. Good leaving groups are weak bases, such as halides (I⁻ > Br⁻ > Cl⁻ > F⁻), tosylates, and mesylates.
The Volkswagen Analogy: A Visual Aid for Understanding
The "Volkswagen" analogy is a helpful mnemonic to visualize the SN2 reaction mechanism. Imagine the Volkswagen as the substrate, with the leaving group (e.g., a halide) acting as the rear bumper. The nucleophile represents a collision from the backside. The reaction proceeds as follows:
- The Approach: The nucleophile approaches the carbon atom from the backside, opposite the leaving group. This is crucial because it allows the nucleophile to simultaneously bond to the carbon atom and the leaving group to depart.
- The Collision: The nucleophile collides with the carbon atom, breaking the bond between the carbon and the leaving group and forming a new bond between the carbon and the nucleophile.
- The Inversion: The entire process results in an inversion of configuration, much like flipping a Volkswagen. The rear bumper (leaving group) is replaced, and the orientation of the other groups on the carbon atom is reversed.
Why the Volkswagen?
The Volkswagen's shape and the image of a rear-end collision offer a clear visualization of the backside attack crucial for the SN2 reaction. The inversion of configuration after the "collision" directly mirrors the stereochemical outcome of the reaction.
Factors Influencing the SN2 Reaction Rate
Several factors influence the rate of the SN2 reaction:
- Strength of the Nucleophile: Stronger nucleophiles, possessing a greater electron density and a stronger tendency to donate electrons, react faster.
- Solvent Effects: Polar aprotic solvents (like DMF, DMSO, acetone) are preferred as they stabilize the transition state and enhance the nucleophile's reactivity. Protic solvents (like water, alcohols) can hinder the reaction by solvating the nucleophile.
- Substrate Structure: Primary substrates react fastest due to less steric hindrance around the reaction center. Secondary substrates react slower, and tertiary substrates generally do not undergo SN2 reactions.
- Leaving Group Ability: Good leaving groups stabilize the negative charge after departure, facilitating the reaction. The stability of the leaving group's conjugate acid is often used to assess its ability to leave.
- Steric Hindrance: Bulky groups around the reaction center hinder the nucleophile's approach, slowing down the reaction.
Comparison with SN1 Reactions
The SN2 reaction contrasts sharply with the SN1 (Substitution Nucleophilic Unimolecular) reaction. In SN1 reactions:
- The reaction proceeds through a two-step mechanism involving a carbocation intermediate.
- The reaction is unimolecular, with the rate-determining step involving only the substrate.
- The reaction is favored by tertiary substrates due to the stability of the tertiary carbocation intermediate.
- Racemization often occurs due to the planar geometry of the carbocation intermediate.
Understanding the difference between SN1 and SN2 reactions is crucial for predicting the outcome of organic reactions.
Applications of the SN2 Reaction in Organic Synthesis
The SN2 reaction is a cornerstone of organic synthesis, employed in countless reactions to build new carbon-carbon and carbon-heteroatom bonds. Some examples include:
- Alkylation of Alcohols: Converting alcohols into ethers or alkyl halides.
- Synthesis of Ethers: Williamson ether synthesis utilizes an SN2 reaction between an alkoxide ion and an alkyl halide.
- Synthesis of Amines: SN2 reaction between an alkyl halide and an amine to form a new C-N bond.
- Synthesis of Nitriles: SN2 reaction between an alkyl halide and a cyanide ion to form a nitrile.
Advanced Topics and Considerations
Ambident Nucleophiles: Some nucleophiles possess two nucleophilic sites. For example, the cyanide ion (CN⁻) can react through either the carbon or the nitrogen atom, leading to different products. The regioselectivity of the reaction depends on factors such as steric hindrance and solvent effects.
Phase-Transfer Catalysis: This technique enhances the reaction rate of SN2 reactions involving water-insoluble reactants by using a phase-transfer catalyst to transfer the nucleophile from the aqueous phase to the organic phase.
Conclusion: Mastering the SN2 Reaction
The SN2 reaction is a cornerstone of organic chemistry, fundamental to understanding and predicting the outcome of numerous reactions. By grasping the concerted mechanism, stereochemical implications, and the influencing factors, one can effectively utilize this versatile tool in organic synthesis. The "Volkswagen" analogy provides a valuable visual aid for remembering the crucial aspects of the reaction, particularly the backside attack and the inversion of configuration. Remember, mastering the SN2 reaction requires a comprehensive understanding of its mechanism and the factors governing its rate and selectivity, paving the way for efficient and predictable synthesis. Further exploration of the related SN1 reaction and the factors that govern the competition between SN1 and SN2 reactions will enhance your capabilities as an organic chemist. The applications of SN2 reactions are incredibly diverse, underscoring its importance in modern chemical synthesis. Continued study and practice will solidify your understanding of this critical reaction mechanism and its applications in organic chemistry.
Latest Posts
Latest Posts
-
I Am Malala Summary By Chapter
Mar 03, 2025
-
Digging Deeper Survival Needs Answer Key
Mar 03, 2025
-
An Atomic Assault Additional Practice Answers
Mar 03, 2025
-
Summary Of Rapunzel By Brothers Grimm
Mar 03, 2025
-
Topic 1 Assessment Form A Answer Key
Mar 03, 2025
Related Post
Thank you for visiting our website which covers about Question Volkswagen Draw The Major Sn2 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
