Reviewing The Concepts Enzymes Answer Key
Onlines
Mar 15, 2025 · 6 min read
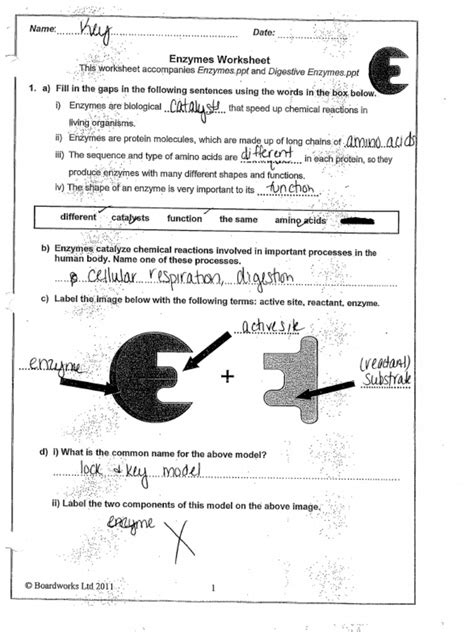
Table of Contents
Reviewing the Concepts: Enzymes – Answer Key and Deep Dive
Enzymes are biological catalysts, crucial for virtually every biochemical reaction within living organisms. Understanding their function, properties, and regulation is fundamental to grasping the complexities of life itself. This comprehensive guide serves as an answer key to common enzyme-related questions, providing a deep dive into the core concepts, backed by explanations and examples. We'll explore topics such as enzyme structure, function, kinetics, inhibition, and their widespread applications.
Enzyme Structure and Function: The Key to Catalysis
What are enzymes? Enzymes are predominantly proteins (though some RNA molecules also exhibit catalytic activity, known as ribozymes) that accelerate the rate of biochemical reactions without being consumed themselves. Their remarkable specificity ensures that they act only on particular substrates, leading to the formation of specific products.
How do enzymes work? Enzymes work by lowering the activation energy of a reaction. This means they provide an alternative reaction pathway with a lower energy barrier, making it easier for the reaction to proceed. They achieve this by binding to their substrates at a specific site called the active site. This interaction forms an enzyme-substrate complex, facilitating the conversion of substrate to product.
Key features of enzyme structure impacting function:
- Active Site: The region where the substrate binds. Its three-dimensional structure is crucial for substrate specificity. Often involves amino acid side chains that interact with the substrate through hydrogen bonds, ionic interactions, hydrophobic interactions, etc.
- Allosteric Site: A site distinct from the active site, which can bind regulatory molecules (allosteric effectors). Binding at the allosteric site can alter the enzyme's shape and activity.
- Cofactors and Coenzymes: Non-protein components essential for enzyme activity. Cofactors are inorganic ions (e.g., Mg²⁺, Zn²⁺), while coenzymes are organic molecules (e.g., NAD⁺, FAD).
Enzyme Specificity: A Lock and Key Model (and its limitations)
The early lock and key model proposed that the enzyme's active site had a rigid, complementary shape to its substrate, like a lock and key. While a useful simplification, this model doesn't fully explain the flexibility observed in enzyme-substrate interactions.
The more accurate induced fit model suggests that the enzyme's active site is flexible and undergoes conformational changes upon substrate binding. This interaction optimizes the orientation and interactions between the enzyme and substrate, facilitating catalysis.
Enzyme Kinetics: Measuring the Rate of Reactions
Factors influencing enzyme activity:
- Substrate Concentration: At low substrate concentrations, the reaction rate increases proportionally. However, at high substrate concentrations, the rate plateaus as the enzyme becomes saturated.
- Temperature: Enzymes have an optimal temperature for maximum activity. Higher temperatures can denature the enzyme (loss of tertiary structure), while lower temperatures slow down the reaction rate.
- pH: Enzymes also have an optimal pH. Changes in pH can alter the ionization state of amino acid residues in the active site, impacting substrate binding and catalysis.
- Enzyme Concentration: Increasing enzyme concentration generally increases the reaction rate, provided sufficient substrate is available.
Michaelis-Menten Kinetics: This model describes the relationship between substrate concentration and reaction rate. It defines two important parameters:
- Km (Michaelis constant): Represents the substrate concentration at which the reaction rate is half of its maximum velocity (Vmax). A lower Km indicates higher affinity of the enzyme for its substrate.
- Vmax (Maximum velocity): The maximum rate of the reaction achieved when the enzyme is saturated with substrate.
Lineweaver-Burk Plot: A Visual Tool for Kinetics
The Lineweaver-Burk plot is a graphical representation of the Michaelis-Menten equation. It transforms the hyperbolic curve into a linear plot, facilitating the determination of Km and Vmax from the x- and y-intercepts.
Enzyme Inhibition: Controlling Enzyme Activity
Enzyme inhibitors are molecules that bind to enzymes and reduce their activity. They are crucial for regulating metabolic pathways and are also valuable as therapeutic agents. There are two main types:
1. Reversible Inhibition: The inhibitor binds non-covalently to the enzyme and can be readily removed. There are three main subtypes:
- Competitive Inhibition: The inhibitor competes with the substrate for binding to the active site. Increasing substrate concentration can overcome this inhibition.
- Uncompetitive Inhibition: The inhibitor binds only to the enzyme-substrate complex, preventing the formation of product.
- Non-competitive Inhibition: The inhibitor binds to a site other than the active site (allosteric site), causing a conformational change that reduces enzyme activity. Substrate concentration does not affect this inhibition.
2. Irreversible Inhibition: The inhibitor binds covalently to the enzyme, permanently inactivating it. This type of inhibition is often used as a mechanism for designing drugs that target specific enzymes.
Enzyme Regulation: Maintaining Cellular Balance
Cells regulate enzyme activity to maintain metabolic homeostasis. Several mechanisms are employed:
- Allosteric Regulation: Binding of regulatory molecules (allosteric effectors) at allosteric sites alters the enzyme's conformation and activity. This can either activate or inhibit the enzyme.
- Covalent Modification: Chemical modification of the enzyme (e.g., phosphorylation, glycosylation) can alter its activity. Phosphorylation is a common regulatory mechanism, often catalyzed by kinases and phosphatases.
- Proteolytic Cleavage: Some enzymes are synthesized as inactive precursors (zymogens) and activated by proteolytic cleavage. This is an irreversible form of activation.
- Gene Regulation: The amount of enzyme produced can be controlled at the transcriptional and translational levels. This long-term regulation adjusts the overall enzyme concentration within the cell.
Enzyme Classification: A Systematic Approach
Enzymes are classified into six main classes based on the type of reaction they catalyze:
- Oxidoreductases: Catalyze oxidation-reduction reactions.
- Transferases: Catalyze the transfer of functional groups.
- Hydrolases: Catalyze hydrolysis reactions (breaking bonds with water).
- Lyases: Catalyze the addition or removal of groups to form double bonds.
- Isomerases: Catalyze isomerization reactions (rearrangement of atoms within a molecule).
- Ligases: Catalyze the joining of two molecules, often coupled with ATP hydrolysis.
Each class is further subdivided into subclasses based on the specific substrates and reactions involved. This system provides a logical and organized framework for understanding the diverse roles of enzymes in biological systems.
Applications of Enzymes: Beyond the Cell
The remarkable catalytic power and specificity of enzymes have led to their widespread applications in various fields:
- Medicine: Enzymes are used in diagnostics (e.g., enzyme-linked immunosorbent assay, ELISA), therapeutics (e.g., treatment of genetic disorders), and as drug targets.
- Industry: Enzymes are used in various industrial processes, such as food processing, textile manufacturing, and biofuel production. Examples include using proteases in detergents or amylases in starch processing.
- Agriculture: Enzymes are used to improve crop yields and quality. For instance, cellulases can break down plant cell walls to enhance nutrient availability.
- Environmental Science: Enzymes play a role in bioremediation, which involves using enzymes to degrade pollutants.
Conclusion: A Deeper Understanding of Life's Catalysts
Enzymes are indispensable for life, driving a multitude of biochemical reactions that sustain cellular function. Understanding their structure, function, kinetics, regulation, and applications is crucial for advancements in various scientific and technological fields. From the intricate mechanisms of enzyme catalysis to their widespread use in biotechnology, enzymes continue to fascinate and inspire researchers across disciplines. This comprehensive overview offers a solid foundation for delving further into the captivating world of these biological workhorses. Further exploration into specific enzyme families, their regulation in different metabolic pathways, and the ever-evolving applications in biotechnology, promises a continued unfolding of the immense contributions of enzymes to our understanding of life and its processes.
Latest Posts
Latest Posts
-
Advanced Hardware Lab 6 4 Troubleshoot Monitors And Video
Mar 15, 2025
-
Murder On The Orient Express Chapter Summary
Mar 15, 2025
-
As Added Texture Expands The Form
Mar 15, 2025
-
2 03 Quiz Symbols And Imagery And Mood And Emotion
Mar 15, 2025
-
Empareja Las Palabras De Forma Logica
Mar 15, 2025
Related Post
Thank you for visiting our website which covers about Reviewing The Concepts Enzymes Answer Key . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
