Section 8.3 Properties Of Acids And Bases
Onlines
Mar 16, 2025 · 7 min read
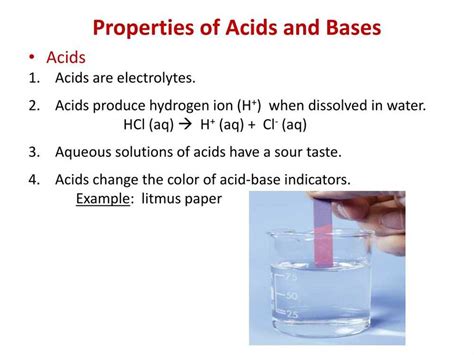
Table of Contents
Section 8.3: Properties of Acids and Bases – A Deep Dive
Understanding the properties of acids and bases is fundamental to chemistry. This comprehensive guide delves into Section 8.3, exploring the characteristic behaviours of these crucial chemical species, their interactions, and the implications of their properties in various applications. We'll move beyond simple definitions, examining the nuances of their behaviour and how these properties are utilized in everyday life and advanced scientific pursuits.
Identifying Acids and Bases: A Look Beyond the Definitions
Before diving into their specific properties, let's briefly revisit the core definitions of acids and bases. While several definitions exist (Arrhenius, Brønsted-Lowry, and Lewis), the most commonly encountered in introductory chemistry are the Arrhenius and Brønsted-Lowry definitions.
Arrhenius Definition:
The Arrhenius definition defines acids as substances that increase the concentration of hydronium ions (H₃O⁺) when dissolved in water, and bases as substances that increase the concentration of hydroxide ions (OH⁻) when dissolved in water. This definition, while simple, is limited as it only applies to aqueous solutions.
Brønsted-Lowry Definition:
The Brønsted-Lowry definition offers a broader perspective. It defines acids as proton (H⁺) donors and bases as proton acceptors. This definition encompasses a wider range of substances and reactions, including those that don't necessarily involve water.
Key Properties of Acids
Acids exhibit a range of characteristic properties, many stemming from their ability to donate protons. Let's explore these properties in detail:
1. Taste:
Acids typically have a sour taste. This is a characteristic property often used to identify acids, though caution is advised, as tasting unknown chemicals can be dangerous. Common examples of sour-tasting foods containing acids include lemons (citric acid) and vinegar (acetic acid).
2. pH:
Acids have a pH less than 7. The pH scale, ranging from 0 to 14, measures the concentration of hydrogen ions (H⁺) in a solution. A lower pH indicates a higher concentration of H⁺, signifying a stronger acid. This property is crucial for understanding acid strength and reactivity. Strong acids, like hydrochloric acid (HCl), have a pH close to 0, while weak acids, such as acetic acid (CH₃COOH), have a higher pH closer to 7.
3. Reaction with Metals:
Many acids react with active metals (like zinc, magnesium, and iron) to produce hydrogen gas (H₂) and a salt. This is a characteristic reaction used to identify acids and is a fundamental concept in redox chemistry. For instance, the reaction between hydrochloric acid and zinc produces hydrogen gas and zinc chloride:
2HCl(aq) + Zn(s) → ZnCl₂(aq) + H₂(g)
4. Reaction with Metal Carbonates and Bicarbonates:
Acids react with metal carbonates and bicarbonates to produce carbon dioxide gas (CO₂), water, and a salt. This reaction is often used as a test for the presence of carbonates and bicarbonates, and also to produce carbon dioxide in certain applications. For example, the reaction between hydrochloric acid and sodium carbonate produces carbon dioxide, water, and sodium chloride:
2HCl(aq) + Na₂CO₃(s) → 2NaCl(aq) + H₂O(l) + CO₂(g)
5. Reaction with Bases (Neutralization):
Acids react with bases in a process called neutralization. This reaction results in the formation of water and a salt. The heat released during this reaction is often significant and can be used to determine the enthalpy change of the neutralization. This principle is fundamental to acid-base titrations, a common analytical technique. For example:
HCl(aq) + NaOH(aq) → NaCl(aq) + H₂O(l)
6. Conductivity:
Strong acids are good conductors of electricity because they dissociate completely in water, producing a high concentration of ions (H₃O⁺ and the conjugate base anion). Weak acids, however, are poor conductors due to their partial dissociation.
7. Indicators:
Acid-base indicators, such as litmus paper and phenolphthalein, change colour depending on the pH of the solution. Acids turn blue litmus paper red and leave phenolphthalein colourless. This property is widely used in qualitative analysis to determine the acidity or basicity of a solution.
Key Properties of Bases
Bases, like acids, exhibit distinct properties, primarily due to their ability to accept protons or release hydroxide ions. Let's examine these properties:
1. Taste:
Bases typically have a bitter taste and a slippery or soapy feel. Again, tasting unknown chemicals is strongly discouraged due to potential hazards. Many household cleaning products, like soaps and detergents, contain bases.
2. pH:
Bases have a pH greater than 7. A higher pH indicates a lower concentration of H⁺ ions and a higher concentration of OH⁻ ions. Strong bases, like sodium hydroxide (NaOH), have a pH close to 14, while weak bases have a pH closer to 7.
3. Reaction with Acids (Neutralization):
Bases react with acids in a neutralization reaction, producing water and a salt, as discussed earlier. This reaction is fundamental to many industrial processes and analytical techniques.
4. Reaction with Fats and Oils (Saponification):
Strong bases, particularly those in aqueous solutions, react with fats and oils in a process called saponification. This reaction produces soap and glycerol. This process is historically significant and still used in soap making.
5. Conductivity:
Strong bases, like strong acids, are good conductors of electricity due to their complete dissociation in water, yielding a high concentration of ions (OH⁻ and the conjugate acid cation). Weak bases are poor conductors.
6. Indicators:
Bases turn red litmus paper blue and turn phenolphthalein pink. This colour change is utilized in titrations and qualitative tests.
Comparing Acid and Base Strengths: A Matter of Degree
The strength of an acid or base refers to its extent of dissociation or ionization in water. Strong acids and bases completely dissociate, while weak acids and bases only partially dissociate. This difference significantly impacts their reactivity and pH.
Strong Acids:
- Completely dissociate in water.
- High concentration of H₃O⁺ ions.
- Low pH values.
- Examples: HCl, H₂SO₄, HNO₃.
Weak Acids:
- Partially dissociate in water.
- Low concentration of H₃O⁺ ions.
- Higher pH values (closer to 7) compared to strong acids.
- Examples: CH₃COOH (acetic acid), HF (hydrofluoric acid).
Strong Bases:
- Completely dissociate in water.
- High concentration of OH⁻ ions.
- High pH values.
- Examples: NaOH, KOH, Ca(OH)₂.
Weak Bases:
- Partially dissociate in water.
- Low concentration of OH⁻ ions.
- Lower pH values (closer to 7) compared to strong bases.
- Examples: NH₃ (ammonia).
Applications of Acids and Bases
The properties of acids and bases make them essential in numerous applications across various fields:
Industrial Applications:
- Manufacturing: Acids and bases are crucial in various manufacturing processes, including the production of fertilizers, plastics, and textiles.
- Metal Processing: Acids are used in metal cleaning, etching, and pickling.
- Food and Beverage Industry: Acids are used as preservatives and flavour enhancers, while bases are used in baking and food processing.
- Petroleum Refining: Acids and bases play critical roles in refining petroleum products.
Everyday Life Applications:
- Cleaning Products: Many household cleaners contain acids or bases for their cleaning and disinfecting properties.
- Food and Drinks: Many foods and drinks naturally contain acids (citrus fruits, vinegar) or bases (baking soda).
- Medicine: Acids and bases are used in pharmaceuticals and drug delivery systems.
- Agriculture: Acids and bases are crucial for soil pH adjustment and fertilizer production.
Conclusion: The Significance of Understanding Acid-Base Properties
The properties of acids and bases are fundamental concepts in chemistry with far-reaching implications. From everyday applications like cleaning and cooking to sophisticated industrial processes and scientific research, understanding their behaviour and interactions is essential. This deep dive into Section 8.3 highlights the importance of comprehending not only the definitions but also the practical implications of these fundamental chemical species. By understanding these properties, we can better appreciate their roles in various aspects of our lives and the world around us. Further exploration into topics like acid-base titrations, pH calculations, and buffer solutions will provide even deeper insights into the fascinating world of acids and bases.
Latest Posts
Latest Posts
-
Elige La Palabra Adecuada Para Completar Las Oraciones Comparativas
Mar 17, 2025
-
You Seem Pleased To Have Successfully Whored Yourself Manacled
Mar 17, 2025
-
Muchas Ninas Jovenes Han Estado A Dieta Terrible
Mar 17, 2025
-
Examples Of Public Data Collected By Law From Physicians Include
Mar 17, 2025
-
Alcohol And Its Effects On The Body Worksheet Answers
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Section 8.3 Properties Of Acids And Bases . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
