The Chlorination Of Propane Proceeds As A Radical Chain Reaction
Onlines
Mar 28, 2025 · 6 min read
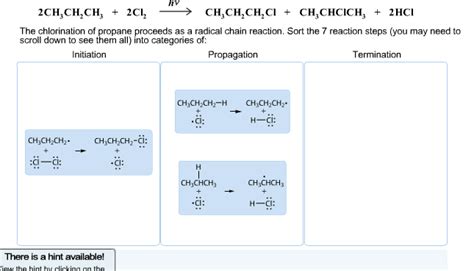
Table of Contents
The Chlorination of Propane: A Deep Dive into Radical Chain Reactions
The chlorination of propane, a seemingly simple organic reaction, provides a fascinating window into the world of free radical chemistry. This process, far from being a straightforward substitution, unfolds as a complex radical chain reaction, involving several distinct steps and exhibiting characteristics that make it a staple in organic chemistry education and industrial applications. This article will delve into the intricacies of this reaction, exploring its mechanism, influencing factors, and its broader significance in the realm of organic chemistry.
Understanding Free Radical Reactions
Before embarking on the specifics of propane chlorination, let's establish a foundational understanding of free radical reactions. These reactions revolve around the participation of free radicals, highly reactive species possessing an unpaired electron. This unpaired electron makes free radicals highly unstable and prone to reacting to achieve a more stable, paired electron configuration. Their reactivity is what drives the chain reaction mechanism.
Key Characteristics of Free Radical Reactions
Free radical reactions are typically characterized by several key features:
- Chain initiation: This stage involves the formation of free radicals, often through homolytic bond cleavage (breaking a bond where each atom receives one electron). This is usually achieved via high temperatures or UV light.
- Chain propagation: This is the heart of the reaction, where the initial free radicals react with other molecules to generate new free radicals, continuing the chain. This step is responsible for the bulk of product formation.
- Chain termination: This stage involves the combination of two free radicals to form a stable molecule, effectively stopping the chain reaction. This can occur through various radical-radical combinations.
- Non-selective nature: Free radical reactions are often non-selective, meaning multiple products can be formed. This is particularly evident in the chlorination of propane, as we shall see.
The Mechanism of Propane Chlorination
The chlorination of propane is a classic example of a free radical substitution reaction. The overall reaction can be represented as:
C₃H₈ + Cl₂ → C₃H₇Cl + HCl
However, this simplified equation masks the complex multi-step mechanism. Let's break down each stage:
1. Chain Initiation: Generating the First Radicals
The reaction is initiated by the homolytic cleavage of a chlorine molecule (Cl₂), typically driven by ultraviolet (UV) light or heat. This generates two chlorine radicals:
Cl₂ ---(UV light or heat)--> 2 Cl•
The dot (•) represents the unpaired electron, characteristic of a free radical. This is the crucial first step in setting off the chain reaction.
2. Chain Propagation: The Reaction Cascade
This stage comprises two main propagation steps:
- Step 1: A chlorine radical abstracts a hydrogen atom from propane, forming a propyl radical and hydrogen chloride:
Cl• + C₃H₈ → C₃H₇• + HCl
This step is crucial as it generates a new radical, the propyl radical (C₃H₇•), which will continue the chain. Note that this hydrogen abstraction can occur at any of the carbons in propane, leading to a mixture of products.
- Step 2: The propyl radical reacts with another chlorine molecule, forming chloropropane and another chlorine radical:
C₃H₇• + Cl₂ → C₃H₇Cl + Cl•
This step regenerates the chlorine radical, which can then participate in another cycle of propagation, sustaining the chain reaction. This cyclical nature explains the chain's name.
3. Chain Termination: Halting the Reaction
The chain reaction continues until two radicals collide and combine to form a stable molecule. Several termination steps are possible:
- Cl• + Cl• → Cl₂
- C₃H₇• + C₃H₇• → C₆H₁₄ (hexane)
- Cl• + C₃H₇• → C₃H₇Cl
These termination steps remove active radicals from the system, thereby stopping the chain reaction. The probability of termination increases as the concentration of radicals rises.
Regioselectivity in Propane Chlorination: A Mixture of Products
The chlorination of propane doesn't yield a single product. The reaction leads to a mixture of 1-chloropropane and 2-chloropropane, due to the possibility of hydrogen abstraction from either primary (CH₃) or secondary (CH₂) carbon atoms.
- 1-chloropropane: This is formed when a chlorine radical abstracts a hydrogen atom from a primary carbon.
- 2-chloropropane: This is formed when a chlorine radical abstracts a hydrogen atom from a secondary carbon.
The ratio of 1-chloropropane to 2-chloropropane is not 1:1. Secondary hydrogens are abstracted more readily than primary hydrogens because secondary carbon radicals are more stable (due to hyperconjugation). Therefore, 2-chloropropane is the major product. However, the exact ratio is influenced by reaction conditions, such as temperature and concentration.
Factors Affecting Propane Chlorination
Several factors significantly impact the outcome of propane chlorination:
- Temperature: Higher temperatures generally lead to faster reaction rates, increasing the chances of multiple chlorination reactions, resulting in products with more than one chlorine atom.
- Concentration of Reactants: The relative concentrations of propane and chlorine influence the reaction rate and the distribution of products. Excess chlorine can lead to polychlorinated products.
- Light Intensity (UV Light): The intensity of UV light directly impacts the rate of chain initiation, influencing the overall reaction rate.
- Presence of Inhibitors: Certain substances, known as inhibitors, can react with free radicals, effectively interrupting the chain reaction and slowing down the process.
Industrial Significance and Applications
The chlorination of propane, while seemingly a simple reaction, finds significant applications in industrial settings. The resultant chloropropanes are versatile intermediates in the synthesis of various organic compounds, including:
- Solvents: Chloropropanes are used as solvents in various industrial processes.
- Refrigerants: Historically, some chloropropanes were employed as refrigerants, although their use is now largely restricted due to environmental concerns.
- Polymer Synthesis: Chloropropanes serve as building blocks for the synthesis of certain polymers.
- Pharmaceuticals: Derivatives of chloropropanes might find application in pharmaceutical manufacturing.
Comparing Propane Chlorination with Other Halogenations
While chlorination is discussed here, it's crucial to acknowledge that propane can also undergo bromination, iodination, and fluorination. However, these reactions differ significantly in their reactivity and selectivity. Bromination, for example, is more selective than chlorination, favoring the formation of the more stable secondary bromide. Fluorination is highly exothermic and often uncontrollable, while iodination is typically very slow and requires specific conditions. Chlorination holds a middle ground in terms of reactivity and selectivity, making it a practically useful reaction for various applications.
Safety Considerations
Working with chlorine gas and free radical reactions requires meticulous safety precautions:
- Chlorine Gas Handling: Chlorine is a toxic and corrosive gas. Appropriate handling procedures, including working in well-ventilated areas and using appropriate personal protective equipment (PPE), are essential.
- UV Light Safety: Exposure to intense UV light should be minimized. Appropriate shielding and eye protection are necessary.
- Waste Disposal: Proper disposal of waste materials is crucial to prevent environmental contamination.
Conclusion: A Versatile Reaction with Broad Implications
The chlorination of propane serves as a prime example of a radical chain reaction, showcasing the intricate mechanisms and diverse factors influencing free radical chemistry. Its industrial significance, versatility in producing various chloropropane isomers, and the underlying principles of free radical reactions make it a pivotal topic in organic chemistry. Understanding this reaction provides a foundational understanding of a vast class of organic transformations, impacting diverse fields ranging from industrial synthesis to environmental chemistry. The careful consideration of reaction conditions, coupled with a thorough understanding of the mechanism, is crucial for controlling the reaction and obtaining the desired products safely and efficiently. The ongoing research and development in this area continue to reveal new possibilities and applications for this fundamental organic reaction.
Latest Posts
Latest Posts
-
Which Diagram Correctly Shows The Events Of The Pueblo Revolt
Mar 31, 2025
-
Alan Smith Physical Therapist Kansas Npi Number
Mar 31, 2025
-
Practice Complex Inheritance Patterns Answer Key
Mar 31, 2025
-
The Guest Summary By Albert Camus
Mar 31, 2025
-
The Great Gatsby Student Workbook Answer Key Pdf
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about The Chlorination Of Propane Proceeds As A Radical Chain Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
