Unit Chemical Bonding Covalent Bonding - Ws #3
Onlines
Mar 10, 2025 · 6 min read
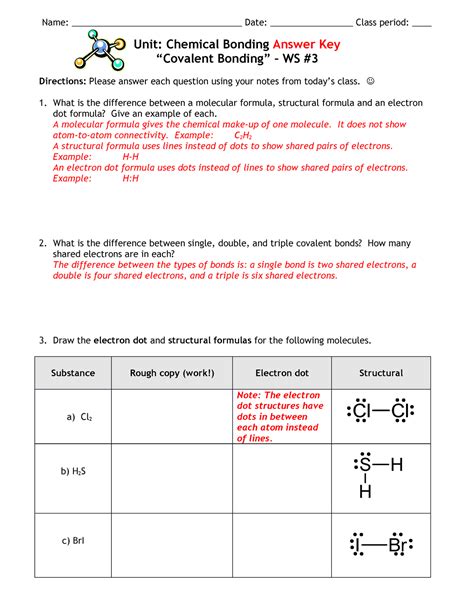
Table of Contents
Unit Chemical Bonding: Covalent Bonding - WS #3: A Deep Dive
This comprehensive guide delves into the intricacies of covalent bonding, a crucial concept in chemistry. We'll explore its fundamental principles, variations, and applications, aiming to provide a robust understanding suitable for students tackling worksheet #3 (or equivalent) in their unit on chemical bonding. We will also cover relevant aspects of VSEPR theory and molecular geometry, crucial for predicting the three-dimensional structure of covalently bonded molecules.
What is Covalent Bonding?
Covalent bonding is a type of chemical bond where two atoms share one or more pairs of electrons to achieve a more stable electron configuration. This sharing occurs between non-metal atoms, often those with high electronegativity, which means they have a strong attraction for electrons. Unlike ionic bonding, where electrons are transferred, covalent bonding involves a mutual sharing of electrons. This sharing leads to the formation of a strong attractive force that holds the atoms together, forming a molecule.
Key Characteristics of Covalent Bonds:
- Sharing of electrons: The defining characteristic of a covalent bond.
- Non-metal atoms: Typically formed between two or more non-metal atoms.
- Lower melting and boiling points: Compared to ionic compounds, covalent compounds generally have lower melting and boiling points. This is because the intermolecular forces (forces between molecules) are weaker than the strong electrostatic forces in ionic compounds.
- Poor conductors of electricity: Covalent compounds usually do not conduct electricity because they lack freely moving charged particles (ions or electrons). Exceptions exist, notably in molten or aqueous solutions of certain polar covalent compounds.
- Formation of molecules: Covalent bonding leads to the formation of discrete molecules with specific shapes and properties.
Types of Covalent Bonds:
Covalent bonds aren't all created equal. Several variations exist, categorized based on the number of electron pairs shared and the nature of the electron sharing:
1. Single Covalent Bonds:
A single covalent bond involves the sharing of one pair of electrons (two electrons) between two atoms. This is represented by a single line (-) in a Lewis structure. For example, the bond in hydrogen gas (H₂) is a single covalent bond: H-H.
2. Double Covalent Bonds:
A double covalent bond involves the sharing of two pairs of electrons (four electrons) between two atoms. This is represented by a double line (=) in a Lewis structure. A classic example is the bond in oxygen gas (O₂): O=O. Double bonds are stronger and shorter than single bonds.
3. Triple Covalent Bonds:
A triple covalent bond involves the sharing of three pairs of electrons (six electrons) between two atoms. This is represented by a triple line (≡) in a Lewis structure. Nitrogen gas (N₂) is a prime example: N≡N. Triple bonds are the strongest and shortest type of covalent bond.
4. Coordinate Covalent Bonds (Dative Bonds):
A coordinate covalent bond, also known as a dative bond, is a special type of covalent bond where both electrons in the shared pair originate from the same atom. This often occurs when one atom has a lone pair of electrons and the other atom has an empty orbital. A common example is found in the ammonium ion (NH₄⁺), where the nitrogen atom donates a lone pair to form a coordinate covalent bond with a hydrogen ion (H⁺).
Lewis Structures and Electron Dot Diagrams:
Lewis structures, also known as electron dot diagrams, are visual representations of covalent bonds. They show the arrangement of valence electrons (outermost electrons) in a molecule. Creating accurate Lewis structures is crucial for understanding the bonding in a molecule. The steps generally involve:
- Counting valence electrons: Determine the total number of valence electrons from all atoms in the molecule. Remember to account for the charge if the molecule is an ion.
- Identifying the central atom: Usually the least electronegative atom (except hydrogen, which is always terminal).
- Connecting atoms with single bonds: Form single bonds between the central atom and other atoms.
- Distributing remaining electrons: Place the remaining electrons around the atoms to satisfy the octet rule (eight electrons in the valence shell for most atoms, except hydrogen, which requires two).
- Forming multiple bonds (if necessary): If some atoms lack an octet, form double or triple bonds to fulfill the octet rule.
- Formal Charge Calculation (if necessary): This step helps determine the most stable Lewis structure among several possibilities.
VSEPR Theory and Molecular Geometry:
The Valence Shell Electron Pair Repulsion (VSEPR) theory predicts the three-dimensional shape of molecules based on the repulsion between electron pairs in the valence shell of the central atom. Electron pairs, whether bonding or non-bonding (lone pairs), repel each other and try to get as far apart as possible. This repulsion determines the molecular geometry. Common shapes include:
- Linear: Two electron pairs around the central atom (180° bond angle). Example: BeCl₂
- Trigonal planar: Three electron pairs (120° bond angle). Example: BF₃
- Tetrahedral: Four electron pairs (109.5° bond angle). Example: CH₄
- Trigonal bipyramidal: Five electron pairs.
- Octahedral: Six electron pairs.
The presence of lone pairs affects the molecular geometry, even though they don't contribute to bonding. Lone pairs exert stronger repulsive forces than bonding pairs, causing deviations from the ideal bond angles.
Polarity of Covalent Bonds:
The electronegativity difference between atoms involved in a covalent bond determines the polarity of the bond. Electronegativity is the ability of an atom to attract electrons in a chemical bond.
- Nonpolar covalent bonds: Occur when the electronegativity difference between the atoms is very small or zero (e.g., bonds between identical atoms like H-H or Cl-Cl). The electrons are shared equally.
- Polar covalent bonds: Occur when the electronegativity difference is significant. The electrons are shared unequally, resulting in a partial positive charge (δ+) on the less electronegative atom and a partial negative charge (δ-) on the more electronegative atom. Example: H-Cl (hydrogen chloride).
Intermolecular Forces:
Intermolecular forces are forces of attraction between molecules. These forces are weaker than covalent bonds but significantly impact the physical properties of covalent compounds. Types of intermolecular forces include:
- London Dispersion Forces (LDFs): Weakest type, present in all molecules. Caused by temporary fluctuations in electron distribution.
- Dipole-Dipole Forces: Occur between polar molecules. The positive end of one molecule attracts the negative end of another.
- Hydrogen Bonding: A special type of dipole-dipole force involving a hydrogen atom bonded to a highly electronegative atom (N, O, or F). Hydrogen bonds are relatively strong intermolecular forces.
Applications of Covalent Bonding:
Covalent bonding is fundamental to the existence of countless molecules essential to life and various industrial applications.
- Organic Chemistry: The backbone of organic chemistry, which studies carbon-based compounds, relies heavily on covalent bonding. This includes molecules like hydrocarbons, proteins, carbohydrates, and nucleic acids.
- Polymers: Many synthetic polymers, such as plastics and rubbers, are built from long chains of covalently bonded atoms.
- Semiconductors: Many semiconductors, crucial in electronics, involve covalent bonding in their crystal structures (e.g., silicon).
- Pharmaceuticals: Numerous pharmaceuticals are organic molecules held together by covalent bonds.
Conclusion:
Understanding covalent bonding is paramount to mastering fundamental chemistry. This detailed exploration of covalent bonding, including its variations, Lewis structures, VSEPR theory, polarity, and intermolecular forces, provides a comprehensive foundation. By grasping these concepts, you will be well-equipped to tackle any worksheet focusing on covalent bonding and confidently apply this knowledge to more complex chemical systems. Remember to practice drawing Lewis structures and predicting molecular geometries to solidify your understanding. This will not only aid in passing your coursework but also open the door to a deeper appreciation of the world around us at a molecular level.
Latest Posts
Latest Posts
-
Match Each Phrase To The Formed Element It Describes
Mar 10, 2025
-
Symbolism In The Gift Of Magi
Mar 10, 2025
-
Summary Of Chapter 6 Animal Farm
Mar 10, 2025
-
The Grasshopper And The Bell Cricket
Mar 10, 2025
-
For This Graph Mark The Statements That Are True
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about Unit Chemical Bonding Covalent Bonding - Ws #3 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
