What Is The Expected Product Of The Reaction Shown
Onlines
Mar 22, 2025 · 6 min read
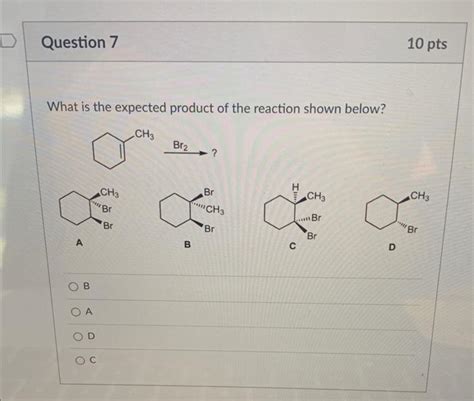
Table of Contents
What is the Expected Product of the Reaction Shown? A Comprehensive Guide
Predicting the product of a chemical reaction is a fundamental skill in chemistry. Understanding reaction mechanisms, functional groups, and reaction conditions is crucial for accurate predictions. This article delves into the process of predicting reaction products, focusing on various reaction types and the factors influencing the outcome. We'll explore strategies for analyzing reaction schemes and identifying the most likely products, considering both major and minor pathways. This detailed guide will empower you to confidently predict reaction products and understand the underlying chemistry.
Understanding Reaction Mechanisms: The Key to Prediction
Before diving into specific examples, it's essential to understand the concept of reaction mechanisms. A reaction mechanism describes the step-by-step process by which reactants transform into products. It involves the breaking and forming of chemical bonds, often involving intermediate species. Knowledge of common reaction mechanisms, such as SN1, SN2, E1, E2, addition, elimination, and oxidation-reduction reactions, is crucial for predicting the products.
Common Reaction Mechanisms and Their Products
-
SN1 (Substitution Nucleophilic Unimolecular): This mechanism involves a two-step process: formation of a carbocation intermediate followed by nucleophilic attack. The carbocation intermediate can undergo rearrangements, leading to unexpected products. SN1 reactions typically favor tertiary substrates and proceed faster in polar protic solvents. Expected product: A substituted product, often with potential for rearrangement.
-
SN2 (Substitution Nucleophilic Bimolecular): This mechanism is a one-step process where the nucleophile attacks the substrate simultaneously with the leaving group departing. SN2 reactions favor primary substrates and are faster in polar aprotic solvents. Expected product: A substituted product with inversion of configuration.
-
E1 (Elimination Unimolecular): This mechanism involves a two-step process: formation of a carbocation intermediate followed by base-induced proton abstraction. Similar to SN1, carbocation rearrangements are possible. E1 reactions are favored by tertiary substrates and proceed faster in polar protic solvents. Expected product: An alkene product, with potential for multiple isomers depending on carbocation stability and base sterics.
-
E2 (Elimination Bimolecular): This mechanism is a one-step process where the base abstracts a proton and the leaving group departs simultaneously. E2 reactions are favored by strong bases and can lead to stereospecific products (Zaitsev's rule often applies, predicting the more substituted alkene). Expected product: An alkene product, often the most substituted isomer (Zaitsev's product).
-
Addition Reactions: These reactions involve the addition of one or more molecules to a multiple bond (e.g., alkene or alkyne). The type of addition (Markovnikov or anti-Markovnikov) depends on the reactants and reaction conditions. Expected product: A saturated molecule or a molecule with a reduced number of multiple bonds.
-
Elimination Reactions: These reactions involve the removal of atoms or groups from a molecule, often resulting in the formation of a multiple bond. The type of elimination (E1 or E2) depends on the substrate, base, and solvent. Expected product: A molecule with a multiple bond, often an alkene.
-
Oxidation-Reduction Reactions: These reactions involve the transfer of electrons. Oxidations involve the loss of electrons, while reductions involve the gain of electrons. The expected product will depend on the oxidizing or reducing agent and the substrate. Expected product: A molecule with a changed oxidation state.
Factors Influencing Reaction Outcomes
Several factors beyond the reaction mechanism significantly influence the outcome of a chemical reaction:
-
Reactant Structure: The structure of the reactants dictates their reactivity and the possible reaction pathways. Steric hindrance, presence of functional groups, and electronic effects all play a role.
-
Reaction Conditions: Temperature, pressure, solvent, and the presence of catalysts or inhibitors can dramatically alter the reaction pathway and the product distribution.
-
Reagent Stoichiometry: The relative amounts of reactants can influence the extent of the reaction and the formation of different products.
-
Stereochemistry: The spatial arrangement of atoms in the reactants can influence the stereochemistry of the products. Chirality and conformational effects can lead to the formation of diastereomers or enantiomers.
-
Solvent Effects: The solvent can stabilize or destabilize intermediates, influencing the reaction rate and selectivity. Polar protic solvents often favor SN1 and E1 reactions, while polar aprotic solvents favor SN2 and E2 reactions.
Predicting Products: A Step-by-Step Approach
Let's outline a systematic approach to predicting the product(s) of a given reaction:
-
Identify the Functional Groups: Determine the functional groups present in the reactants. This is crucial for identifying potential reaction sites and likely reaction types.
-
Identify the Reaction Type: Based on the functional groups and reagents, determine the most likely reaction type (SN1, SN2, E1, E2, addition, elimination, etc.).
-
Consider the Reaction Mechanism: Understand the detailed steps of the reaction mechanism. This helps anticipate intermediate structures and potential side reactions.
-
Analyze Reagent Reactivity: Assess the reactivity of the reagents. Strong bases favor elimination, while weak bases favor substitution. Strong nucleophiles favor SN2 reactions.
-
Consider Stereochemistry: If chiral centers are involved, analyze the effect of the reaction on stereochemistry (inversion, retention, racemization).
-
Predict Major and Minor Products: Based on the reaction mechanism and the factors mentioned above, predict the major and minor products. Consider factors like carbocation stability, steric hindrance, and Zaitsev's rule.
-
Consider Side Reactions: Anticipate possible side reactions that could compete with the main reaction, leading to byproducts.
Examples of Predicting Reaction Products
Let's illustrate this approach with a few examples:
Example 1: SN2 Reaction
-
Reactants: 1-bromopropane and sodium methoxide (NaOCH3) in methanol.
-
Reaction Type: SN2 reaction (strong nucleophile, primary alkyl halide).
-
Mechanism: The methoxide ion attacks the carbon atom bonded to the bromine, displacing the bromide ion.
-
Expected Product: Methyl propyl ether.
Example 2: E1 Reaction
-
Reactants: 2-bromo-2-methylpropane and ethanol at elevated temperature.
-
Reaction Type: E1 reaction (tertiary alkyl halide, polar protic solvent).
-
Mechanism: The bromide ion leaves, forming a tertiary carbocation. A proton is abstracted from a neighboring carbon, leading to the formation of an alkene.
-
Expected Product: 2-methylpropene (isobutylene).
Example 3: Addition Reaction
-
Reactants: Ethene and bromine (Br2) in dichloromethane.
-
Reaction Type: Electrophilic addition.
-
Mechanism: Bromine adds across the double bond, resulting in a vicinal dibromide.
-
Expected Product: 1,2-dibromoethane.
Conclusion
Predicting the expected product of a chemical reaction requires a comprehensive understanding of reaction mechanisms, functional groups, and reaction conditions. By systematically analyzing the reactants and reagents, considering the likely reaction type, and applying the principles of organic chemistry, one can accurately predict the major and minor products. While some reactions may yield multiple products or unexpected outcomes due to side reactions, mastering this skill is crucial for success in organic chemistry and related fields. This detailed guide, combined with practice and further exploration, will enhance your ability to decipher complex reaction schemes and predict the ultimate outcome of a chemical transformation. Remember to always consider the influence of reaction conditions and the unique properties of each reagent involved to achieve the most accurate predictions. Continuous learning and problem-solving are key to mastering this valuable skill.
Latest Posts
Latest Posts
-
Note Three Motives Behind The European Race
Mar 23, 2025
-
Select All Of The Following Which Describes The Crescent Arrangement
Mar 23, 2025
-
Calculus Early Transcendentals 9th Edition Solutions
Mar 23, 2025
-
Assuming That The First Two Paragraphs
Mar 23, 2025
-
The Grouping Of Gestures Facial Expressions And Postures
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about What Is The Expected Product Of The Reaction Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
