What Is The Likely Product Of The Reaction Shown
Onlines
Mar 23, 2025 · 6 min read
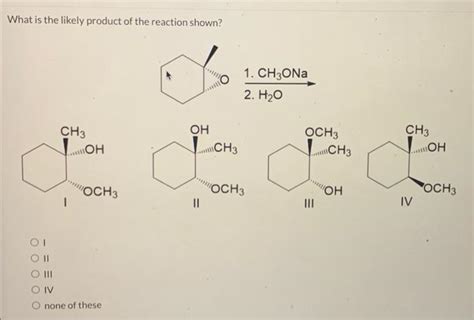
Table of Contents
What is the Likely Product of the Reaction Shown? A Deep Dive into Predicting Reaction Outcomes
Predicting the outcome of a chemical reaction is a cornerstone of chemistry. Understanding reaction mechanisms, functional groups, and reaction conditions is crucial for accurately predicting the product(s) formed. This article explores the process of predicting reaction products, focusing on various reaction types and the factors that influence their outcome. We'll move beyond simple, single-step reactions and delve into more complex scenarios that often arise in organic and inorganic chemistry.
Understanding Reaction Mechanisms: The Key to Prediction
Before diving into specific examples, it's vital to grasp the concept of reaction mechanisms. A reaction mechanism is a step-by-step description of how a reaction proceeds, including the breaking and formation of bonds, the movement of electrons, and the formation of intermediate species. Knowing the mechanism allows us to accurately predict the products because we can trace the transformation of reactants to products through each individual step.
Factors Influencing Reaction Outcomes
Numerous factors can influence the outcome of a chemical reaction. These include:
- Reactants: The nature of the reactants, their functional groups, and their steric properties heavily influence the reaction pathway.
- Reagents: The type and quantity of reagents used can significantly impact the reaction, often dictating the selectivity and yield of the products. Stronger reagents may lead to more extensive reactions.
- Solvent: The choice of solvent can affect reaction rates and selectivity. Polar solvents favor polar reactions, while non-polar solvents favor non-polar reactions.
- Temperature: Temperature affects reaction kinetics. Higher temperatures generally increase reaction rates, but can also lead to unwanted side reactions or decomposition of products.
- Pressure: Pressure plays a significant role in reactions involving gases. Higher pressures often favor reactions that produce fewer gas molecules.
- Catalyst: Catalysts speed up reactions by lowering the activation energy without being consumed in the process. They can also influence reaction selectivity, favoring the formation of specific products.
Predicting Products: A Step-by-Step Approach
Predicting the product(s) of a reaction often involves a systematic approach:
-
Identify the Functional Groups: Determine the functional groups present in the reactants. These groups are the reactive centers of the molecule and will dictate how the molecule participates in a chemical reaction. Examples include alcohols (-OH), carboxylic acids (-COOH), alkenes (C=C), and halides (-X).
-
Recognize the Reaction Type: Classify the reaction type. Common reaction types include addition, substitution, elimination, condensation, oxidation, and reduction. This classification will narrow down the possible reaction pathways.
-
Consider Reaction Conditions: Note the reaction conditions, such as temperature, pressure, solvent, and the presence of catalysts or reagents. These conditions often influence the reaction pathway and the nature of the products.
-
Apply Relevant Reaction Mechanisms: Utilize your knowledge of reaction mechanisms to trace the transformation of reactants to products. This involves understanding the movement of electrons, the formation of intermediate species, and the breaking and forming of bonds.
-
Predict the Products: Based on the above steps, predict the structure and stereochemistry of the products. Consider the possibility of multiple products and the relative yields of each product.
Examples of Predicting Reaction Products
Let's illustrate this process with some examples. Note that without a specific reaction shown, I will provide examples across common reaction types. For a truly accurate prediction, the specific reaction equation is necessary.
Example 1: SN1 Reaction
Consider the reaction of tert-butyl bromide with water. This is a classic SN1 (substitution nucleophilic unimolecular) reaction.
-
Functional Groups: tert-butyl bromide contains a tertiary alkyl halide, and water contains a hydroxyl group.
-
Reaction Type: SN1 reaction.
-
Reaction Conditions: Usually performed in a polar protic solvent like water or ethanol.
-
Mechanism: The reaction proceeds through a carbocation intermediate. The bromide ion leaves, forming a stable tertiary carbocation. Water then attacks the carbocation, forming a tert-butyl alcohol. A subsequent proton transfer yields the final product.
-
Product: The predicted product is tert-butyl alcohol.
Example 2: Addition Reaction (Electrophilic Addition)
Consider the reaction of ethene with bromine. This is an electrophilic addition reaction.
-
Functional Groups: Ethene contains a carbon-carbon double bond. Bromine is a diatomic molecule.
-
Reaction Type: Electrophilic addition.
-
Reaction Conditions: Typically occurs at room temperature in a non-polar solvent like dichloromethane.
-
Mechanism: Bromine adds across the double bond in a concerted mechanism. One bromine atom becomes attached to each carbon atom.
-
Product: The predicted product is 1,2-dibromoethane.
Example 3: Elimination Reaction (E1)
Consider the reaction of 2-bromo-2-methylpropane with a strong base like potassium hydroxide. This is an E1 (elimination unimolecular) reaction.
-
Functional Groups: 2-bromo-2-methylpropane contains a tertiary alkyl halide. Potassium hydroxide is a strong base.
-
Reaction Type: E1 elimination.
-
Reaction Conditions: Typically performed with a strong base in a polar protic solvent, such as ethanol. Heat may also be required.
-
Mechanism: The reaction proceeds through a carbocation intermediate. The bromide ion leaves, forming a tertiary carbocation. A base then abstracts a proton from a carbon adjacent to the carbocation, leading to the formation of a double bond.
-
Product: The major product is 2-methylpropene (isobutylene). Minor products may also be formed due to carbocation rearrangements.
Example 4: Esterification Reaction
Consider the reaction between a carboxylic acid and an alcohol in the presence of an acid catalyst. This is an esterification reaction.
-
Functional Groups: Carboxylic acid (-COOH) and alcohol (-OH).
-
Reaction Type: Condensation Reaction (Specifically Esterification)
-
Reaction Conditions: Acid catalyst (like sulfuric acid) is required, often with heating.
-
Mechanism: The acid catalyst protonates the carbonyl oxygen of the carboxylic acid. The alcohol then attacks the carbonyl carbon, followed by proton transfer steps and elimination of water, leading to the formation of an ester.
-
Product: An ester is formed. For instance, the reaction between acetic acid (CH3COOH) and ethanol (CH3CH2OH) will yield ethyl acetate (CH3COOCH2CH3).
The Importance of Considering Side Reactions
It's crucial to acknowledge that side reactions can occur, leading to the formation of multiple products. Factors like reaction conditions, reactant concentration, and the presence of impurities can influence the formation of side products. Predicting the likelihood of side reactions requires a thorough understanding of the reaction mechanism and the possible competing pathways.
Advanced Techniques for Predicting Reaction Outcomes
More sophisticated techniques, often involving computational chemistry, are used to predict reaction outcomes, particularly in complex reactions. These methods use quantum mechanics or molecular mechanics to model the reaction pathway and calculate energies of reactants, products, and intermediates, providing insights into reaction feasibility and selectivity. These methods allow for the prediction of products even in scenarios where traditional methods fall short.
Conclusion
Predicting the product(s) of a chemical reaction is a multifaceted process that requires a solid understanding of reaction mechanisms, functional groups, and reaction conditions. While simple reactions can often be predicted using established rules and principles, more complex reactions may necessitate the application of advanced computational methods. The ability to accurately predict reaction outcomes is essential for the design and optimization of chemical processes and the development of new chemical compounds. Always remember to carefully consider all factors that might influence the reaction, including the possibility of side reactions. With practice and a systematic approach, accurately predicting reaction products becomes a powerful skill for any chemist.
Latest Posts
Latest Posts
-
The Eye In The Tell Tale Heart
Mar 25, 2025
-
Maria Se Caso Despues De Graduarse
Mar 25, 2025
-
Which Of The Following Professionals Cannot Diagnose A Patient
Mar 25, 2025
-
Chapter Summaries For The Picture Of Dorian Gray
Mar 25, 2025
-
Major Battles Of World War 2 Map Answer Key
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Likely Product Of The Reaction Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
