What Is The Missing Reagent In The Reaction Below
Onlines
Mar 09, 2025 · 5 min read
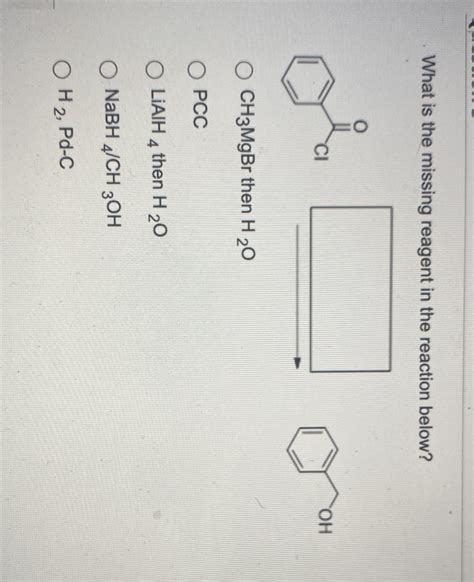
Table of Contents
What's the Missing Reagent? Deciphering Complex Chemical Reactions
Determining the missing reagent in a chemical reaction requires a deep understanding of reaction mechanisms, functional groups, and stoichiometry. This article will delve into the process of identifying missing reagents, using various examples to illustrate different approaches and highlighting the importance of understanding reaction conditions and product formation. We'll focus on organic chemistry reactions, but the underlying principles are applicable across many branches of chemistry.
Understanding the Fundamentals: Reaction Mechanisms and Stoichiometry
Before tackling specific examples, let's review the core concepts:
-
Reaction Mechanism: This outlines the step-by-step process of how reactants transform into products. Understanding the mechanism is crucial because it dictates the type of reagents needed. For example, a nucleophilic substitution reaction requires a nucleophile, while an electrophilic addition reaction needs an electrophile.
-
Stoichiometry: This refers to the quantitative relationships between reactants and products. A balanced chemical equation provides the molar ratios, ensuring that the number of atoms of each element remains the same on both sides of the equation. This is essential for determining the required amount of a missing reagent.
-
Functional Groups: These are specific groups of atoms within a molecule that are responsible for its chemical reactivity. Identifying the functional groups in reactants and products is crucial for understanding the transformation and deducing the necessary reagent.
-
Reaction Conditions: Temperature, pressure, solvent, and the presence of catalysts all influence the reaction outcome. These conditions must be considered when identifying the missing reagent.
Identifying the Missing Reagent: A Step-by-Step Approach
Let's analyze how to identify a missing reagent, using a methodical approach:
-
Analyze the Reactants and Products: Carefully examine the structures of the starting materials (reactants) and the final products. Note the changes in functional groups, the addition or removal of atoms, and any rearrangements in the carbon skeleton. This analysis provides clues about the type of reaction involved (e.g., oxidation, reduction, addition, substitution, elimination).
-
Identify the Reaction Type: Based on the changes observed in step 1, classify the reaction. Is it an addition, substitution, elimination, oxidation, reduction, or a combination of these? This classification significantly narrows down the possibilities for the missing reagent.
-
Determine the Missing Steps: Often, a single reagent doesn't accomplish the entire transformation. The reaction might proceed through several steps, with each step requiring a specific reagent. Identify the intermediate steps and the reagents necessary for each transformation.
-
Consider Reaction Conditions: The reaction conditions—solvent, temperature, pressure, and catalysts—play a crucial role in determining the outcome. Certain reagents are effective only under specific conditions. For instance, a Grignard reagent is typically used in anhydrous ether solvents.
-
Check for Balanced Equations: Once you've identified the potential missing reagent(s), ensure that the overall reaction is stoichiometrically balanced. The number of atoms of each element should be equal on both sides of the equation. Adjusting the stoichiometric coefficients might be necessary.
Examples Illustrating Missing Reagent Identification
Let's consider some examples to solidify the understanding. Note that these examples are simplified and presented to illustrate the principles. Real-world scenarios are often much more complex.
Example 1: Alcohol Oxidation
Let's say we have a primary alcohol as a reactant and a carboxylic acid as the product. The missing reagent is likely an oxidizing agent. Common oxidizing agents for alcohol oxidation include chromic acid (H₂CrO₄), potassium permanganate (KMnO₄), and Jones reagent (CrO₃ in aqueous sulfuric acid). The specific choice depends on the desired selectivity and reaction conditions.
Example 2: Alkene Halogenation
If we have an alkene reactant and a vicinal dihalide product, the missing reagent is likely a halogen (e.g., Cl₂, Br₂, I₂). This reaction is an electrophilic addition where the halogen molecule adds across the double bond.
Example 3: Grignard Reaction
Suppose we have a ketone reactant and a tertiary alcohol product. The missing reagent is likely a Grignard reagent (RMgX), where R is an alkyl or aryl group, and X is a halogen. The Grignard reagent acts as a nucleophile, attacking the carbonyl carbon of the ketone. This is followed by an acidic workup (e.g., aqueous HCl) to protonate the alkoxide intermediate and yield the tertiary alcohol. Remember, Grignard reactions require anhydrous conditions to avoid decomposition of the reagent.
Example 4: Esterification
Starting with a carboxylic acid and an alcohol, the missing reagent for the formation of an ester is an acid catalyst, such as sulfuric acid (H₂SO₄). This is a classic esterification reaction that proceeds through an acid-catalyzed nucleophilic acyl substitution mechanism.
Example 5: Wittig Reaction
If a carbonyl compound (aldehyde or ketone) is converted to an alkene, the missing reagent is likely a Wittig reagent (phosphorus ylide). This reaction involves the formation of a four-membered ring intermediate, followed by its decomposition to yield the alkene and triphenylphosphine oxide.
Advanced Considerations and Challenges
-
Protecting Groups: In complex molecules, protecting groups might be necessary to prevent unwanted reactions on sensitive functional groups. Identifying the need for protecting groups and the appropriate protecting reagents adds another layer of complexity.
-
Regioselectivity and Stereoselectivity: Some reactions can produce multiple products. Understanding regioselectivity (where the reagent adds) and stereoselectivity (which stereoisomer is formed) is crucial for predicting the missing reagent and optimizing the reaction outcome.
-
Multi-Step Synthesis: Complex organic syntheses often involve multiple steps. Identifying the missing reagents in a multi-step synthesis requires a detailed understanding of each reaction step and the overall synthetic strategy.
-
Spectroscopic Data: Nuclear Magnetic Resonance (NMR) and Infrared (IR) spectroscopy, alongside Mass Spectrometry (MS), can provide valuable information about the structure of the reactants, products, and potential intermediates. This information is critical in identifying the missing reagent.
Conclusion: The Art and Science of Reagent Identification
Identifying a missing reagent is a crucial skill in chemistry. It combines both the art of deductive reasoning and the science of understanding reaction mechanisms and stoichiometry. By systematically analyzing the reactants and products, classifying the reaction type, and considering the reaction conditions, you can effectively determine the missing reagent(s) necessary for a given transformation. This is a skill that improves with practice, and by working through numerous examples, you can develop a deep understanding of the principles involved in designing and understanding chemical reactions. Remember to always consult reliable chemical resources and literature when working with chemical reactions and reagents. Safety should always be your top priority.
Latest Posts
Latest Posts
-
Choose The Generative Ai Models For Language From The Following
Mar 09, 2025
-
Chapter Summaries For The Book Thief
Mar 09, 2025
-
An Office Spends 750 A Month On Supplies
Mar 09, 2025
-
Ap Lang Unit 5 Progress Check Mcq
Mar 09, 2025
-
Black Mamas Lives Matter Jada Jones
Mar 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Missing Reagent In The Reaction Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
