What Reagents Are Necessary To Carry Out The Conversion Shown
Onlines
Mar 09, 2025 · 5 min read
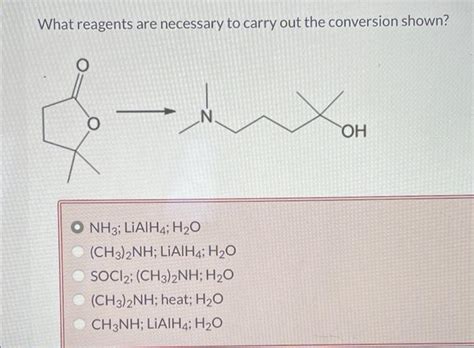
Table of Contents
What Reagents Are Necessary to Carry Out the Conversion Shown? A Comprehensive Guide to Organic Chemistry Reagent Selection
The question of which reagents are necessary to effect a specific organic transformation is central to synthetic organic chemistry. This seemingly simple question often masks a complex interplay of factors, including reaction mechanism, substrate reactivity, selectivity, and yield. This article will delve into the considerations involved in reagent selection, providing a detailed framework for approaching this crucial aspect of organic synthesis. We'll explore several example conversions, highlighting the rationale behind choosing specific reagents and the potential pitfalls to avoid.
Understanding Reaction Mechanisms: The Foundation of Reagent Selection
Before even considering specific reagents, a deep understanding of the reaction mechanism is paramount. The mechanism dictates the steps involved in the transformation, identifying the reactive sites and the intermediates formed. This mechanistic understanding directly informs the choice of reagents. For instance:
-
Nucleophilic Substitution (SN1 and SN2): SN1 reactions favor tertiary alkyl halides and proceed through a carbocation intermediate, making reagents that stabilize carbocations (e.g., silver nitrate) beneficial. SN2 reactions, conversely, prefer primary alkyl halides and proceed via a backside attack, favoring strong nucleophiles (e.g., sodium cyanide, potassium hydroxide) and often aprotic solvents.
-
Elimination Reactions (E1 and E2): E1 reactions, like SN1, proceed through a carbocation intermediate, often favored by high temperatures and the presence of weak bases. E2 reactions, however, require a strong base (e.g., potassium tert-butoxide) and often proceed through an anti-periplanar transition state.
-
Addition Reactions: These reactions involve adding atoms or groups across a multiple bond (alkene or alkyne). Reagents involved depend heavily on the nature of the multiple bond and the desired product. Examples include electrophilic additions (e.g., addition of HBr to an alkene) and nucleophilic additions (e.g., Grignard reagent addition to a carbonyl).
-
Oxidation and Reduction Reactions: These reactions involve changes in oxidation state. Oxidizing agents (e.g., potassium permanganate, chromic acid) increase the oxidation state, while reducing agents (e.g., lithium aluminum hydride, sodium borohydride) decrease it. The specific choice hinges on the functional group being oxidized or reduced and the desired level of oxidation.
Case Studies: Reagent Selection in Action
Let's examine several specific transformations to illustrate the principles outlined above.
Case Study 1: Conversion of an Alkyl Halide to an Alcohol
The conversion of an alkyl halide (RX) to an alcohol (ROH) can be achieved through several pathways, primarily nucleophilic substitution.
Scenario A: Primary Alkyl Halide
For a primary alkyl halide, an SN2 reaction is favored. Suitable reagents include:
-
NaOH or KOH (in aqueous solution): These strong bases act as nucleophiles, replacing the halide with a hydroxide group. The reaction typically proceeds with inversion of configuration.
-
Ag₂O (in aqueous solution): Silver oxide can facilitate the reaction by forming a silver halide precipitate, driving the equilibrium towards product formation.
Scenario B: Tertiary Alkyl Halide
A tertiary alkyl halide favors SN1 conditions, where a carbocation intermediate is formed. Here, a weaker nucleophile is preferable to avoid competing elimination reactions. Suitable reagents include:
- H₂O (in the presence of a weak acid catalyst): Water acts as a weak nucleophile, attacking the carbocation to form the alcohol.
Potential Pitfalls: In both cases, competing elimination reactions are possible, especially at higher temperatures. Careful control of reaction conditions (temperature, solvent) is crucial.
Case Study 2: Conversion of an Alkene to an Epoxide
Epoxidation of alkenes involves the formation of a three-membered cyclic ether (epoxide). Common reagents include:
-
mCPBA (meta-Chloroperoxybenzoic acid): A peroxyacid that acts as an electrophilic oxidant, adding an oxygen atom across the alkene double bond. This is a highly selective reaction, usually proceeding with retention of stereochemistry.
-
Peroxides (e.g., hydrogen peroxide): In the presence of a transition metal catalyst (e.g., titanium(IV) complexes), peroxides can also epoxidize alkenes, often with higher regioselectivity depending on the catalyst.
Potential Pitfalls: Over-oxidation can occur, leading to the formation of other products. Careful control of stoichiometry and reaction conditions is critical.
Case Study 3: Conversion of a Ketone to an Alcohol
The reduction of a ketone to a secondary alcohol is a common transformation. Several reagents are available, each with its own advantages and limitations:
-
NaBH₄ (Sodium borohydride): A mild reducing agent that selectively reduces ketones and aldehydes to alcohols. It's relatively safe and easy to handle.
-
LiAlH₄ (Lithium aluminum hydride): A more powerful reducing agent capable of reducing a wider range of functional groups, including esters, carboxylic acids, and nitriles. It requires careful handling due to its reactivity with water.
Potential Pitfalls: LiAlH₄ is highly reactive and requires anhydrous conditions. Over-reduction can occur if not carefully controlled. NaBH₄ is generally safer but may not be effective for sterically hindered ketones.
Case Study 4: Conversion of an Alcohol to an Alkyl Halide
Converting an alcohol to an alkyl halide typically involves nucleophilic substitution at the hydroxyl group. Common reagents include:
-
PBr₃ (Phosphorus tribromide): Reacts with the alcohol to form the corresponding alkyl bromide.
-
SOCl₂ (Thionyl chloride): Reacts with the alcohol to form the corresponding alkyl chloride.
-
HCl (Hydrochloric acid): Can be used with tertiary alcohols to form the corresponding alkyl chloride, but often requires higher temperatures and longer reaction times.
Potential Pitfalls: Rearrangements can occur during the formation of carbocation intermediates, especially with secondary and tertiary alcohols. Careful selection of reagents and reaction conditions is crucial to minimize rearrangement.
Advanced Considerations: Selectivity and Protecting Groups
In complex syntheses, achieving selectivity—performing a desired transformation without affecting other functional groups—is crucial. This often requires the use of protecting groups, temporary modifications of functional groups to mask their reactivity. Common protecting groups for alcohols include:
-
TBS (tert-butyldimethylsilyl): A bulky silyl ether protecting group.
-
Benzyl ether: A relatively stable ether protecting group.
-
Acetate esters: Relatively easy to introduce and remove.
The choice of protecting group depends on the specific reaction conditions and the other functional groups present in the molecule. Careful planning and execution are necessary to ensure the successful introduction and removal of protecting groups without affecting other parts of the molecule.
Conclusion: A Holistic Approach to Reagent Selection
Selecting the appropriate reagents for an organic transformation requires a comprehensive understanding of reaction mechanisms, substrate reactivity, and potential side reactions. The examples presented here highlight the critical interplay between these factors. Successful synthetic chemists master not only the individual reactions but also the art of strategically choosing reagents that maximize yield, selectivity, and efficiency, often employing protecting groups to achieve the desired outcome in complex molecules. This holistic approach, combined with meticulous experimental design and execution, is essential for achieving success in organic synthesis.
Latest Posts
Latest Posts
-
What Technological Medium Makes Phis Vulnerable To Privacy Breaches
Mar 10, 2025
-
Pain In Fractured Ankle Integral Condition
Mar 10, 2025
-
Unit 6 Test Study Guide Similar Triangles
Mar 10, 2025
-
The Team Will Play First Game On Saturday
Mar 10, 2025
-
Summary Of Chapter 4 Animal Farm
Mar 10, 2025
Related Post
Thank you for visiting our website which covers about What Reagents Are Necessary To Carry Out The Conversion Shown . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
