Which Of The Following Studies Would Need Irb Approval
Onlines
Mar 13, 2025 · 6 min read
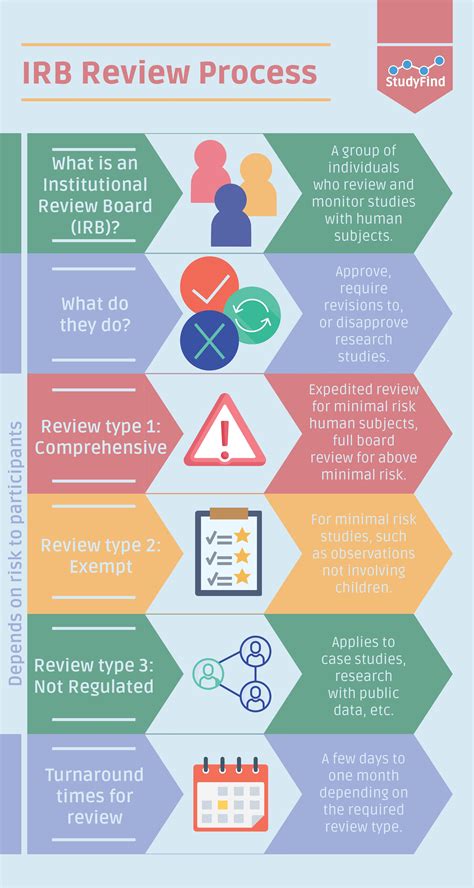
Table of Contents
Which Studies Need IRB Approval? A Comprehensive Guide
Navigating the world of research ethics can be complex, particularly when it comes to determining which studies require Institutional Review Board (IRB) approval. This comprehensive guide aims to clarify the process and provide you with a clear understanding of when IRB review is necessary. We'll delve into the intricacies of IRB regulations, explore various research scenarios, and offer practical examples to help you determine if your study needs IRB approval.
Understanding the Institutional Review Board (IRB)
The IRB is an independent ethics committee that reviews research proposals involving human subjects to ensure the protection of their rights and welfare. Their primary goal is to minimize risks and maximize benefits for participants. This ethical oversight is crucial for maintaining the integrity of research and fostering public trust in the scientific process. IRBs operate under guidelines established by federal regulations, primarily the Common Rule (45 CFR 46).
Key Considerations for IRB Approval
Several factors determine whether your research necessitates IRB approval. These include:
-
Involvement of Human Subjects: This is the most fundamental criterion. If your study involves human participants, even indirectly, IRB review is likely required. "Human subjects" are defined broadly and encompass individuals whose data, biological specimens, or identifiable private information are collected or used in research.
-
Type of Research: The nature of your research plays a significant role. Studies involving interventions, surveys, interviews, or the use of existing data that can identify individuals generally require IRB review. Even observational studies may need approval, depending on the context and potential risks to participants.
-
Level of Risk: The potential risks to participants are carefully evaluated. Studies with minimal risk (e.g., anonymous surveys with low-risk questions) may undergo expedited review, while those with greater risks (e.g., invasive procedures, studies involving vulnerable populations) require full board review.
-
Data Collection Methods: The methods used to collect data directly influence the need for IRB approval. Methods involving direct interaction with participants (interviews, focus groups) necessitate review, while some secondary data analyses might not, although this depends heavily on the nature of the data and its identification potential.
Scenarios Requiring IRB Approval
Let's explore various research scenarios and determine whether IRB approval is needed:
1. Surveys and Questionnaires
Scenario: You're conducting a survey to assess student satisfaction with university services. The survey includes questions about demographics, course experiences, and opinions on campus facilities. Participants are identified by student ID number.
IRB Approval: Yes. This study involves human subjects (students) and collects identifiable private information. Even though the questions are not inherently sensitive, the use of student ID numbers for identification makes IRB approval necessary. This would likely undergo expedited review if the risks are minimal.
Scenario: You're administering an anonymous online survey about general health habits. No identifying information is collected.
IRB Approval: Potentially No. While this involves human subjects, the anonymity of the responses significantly reduces the risk to participants. Some IRBs may still require review, even expedited, while others might deem it exempt. Always check your institution’s specific guidelines.
2. Interviews and Focus Groups
Scenario: You're conducting in-depth interviews with individuals who have experienced a specific type of trauma to understand their coping mechanisms.
IRB Approval: Yes. This research involves direct interaction with participants and explores sensitive topics. IRB approval is mandatory to ensure the participants' emotional well-being and protect their confidentiality. This requires a full board review due to the potential for psychological risk.
Scenario: You're conducting focus groups to gather feedback on a new product design. Participants are compensated for their time.
IRB Approval: Yes. While the risks might be minimal, the interaction with participants and the potential for identifying information necessitates IRB review. The compensation adds another layer of ethical considerations. Expedited review may be possible, depending on the IRB’s assessment.
3. Experiments and Interventions
Scenario: You're conducting a randomized controlled trial testing the effectiveness of a new medication for a specific illness.
IRB Approval: Yes. This study involves a direct intervention, carries potential risks (side effects of medication), and requires rigorous ethical oversight. Full board review is essential.
Scenario: You're conducting a study observing children's behavior in a playground setting without any intervention.
IRB Approval: Potentially Yes. Although there is no direct intervention, the observation of children in a public setting may raise ethical concerns, especially regarding privacy and informed consent. The IRB will carefully assess the level of risk and whether parental consent is required.
4. Secondary Data Analysis
Scenario: You're analyzing publicly available datasets from a government agency, such as census data, which does not contain any personal identifiers.
IRB Approval: Likely No. This type of analysis typically does not require IRB approval because the data is de-identified and publicly available.
Scenario: You're analyzing medical records from a hospital database that contains identifying information, even if you plan to anonymize the data for analysis.
IRB Approval: Yes. Access to protected health information (PHI) is strictly regulated under HIPAA and requires IRB approval, even if the data is anonymized during analysis. The initial access to identifiable data triggers the need for IRB review.
5. Qualitative Research
Scenario: You're conducting ethnographic research, living within a community for an extended period to study their culture.
IRB Approval: Yes. This type of research involves intimate interactions with participants and necessitates careful consideration of ethical implications. Building trust, ensuring confidentiality, and minimizing disruption to the community are crucial elements requiring IRB oversight.
Scenario: You are conducting a literature review based on already published studies.
IRB Approval: No. A literature review does not involve human subjects and therefore does not require IRB approval.
Exemptions and Expedited Review
While many studies require IRB review, some may qualify for exemptions or expedited review. Exemptions are granted for certain types of research that pose minimal risk, such as studies involving publicly available data or educational settings under certain conditions. Expedited review is suitable for studies with minimal risk that do not meet exemption criteria. The specifics of exemptions and expedited review vary across institutions.
International Considerations
Research involving human subjects conducted internationally must adhere to both the ethical guidelines of the researchers’ home institution and the regulations of the country where the research is being conducted. This often involves navigating multiple IRB processes and ensuring compliance with potentially differing ethical standards.
Conclusion: Proactive Approach is Key
Determining whether your study needs IRB approval requires careful consideration of various factors. It is crucial to consult your institution's IRB guidelines and seek clarification when uncertain. A proactive approach, involving early consultation with the IRB, minimizes delays and ensures ethical compliance throughout the research process. Remember, the IRB's role is not to obstruct research but to safeguard the rights and well-being of participants. By understanding and respecting these ethical guidelines, researchers contribute to the advancement of knowledge while upholding the highest standards of research integrity. Always err on the side of caution and seek IRB review when in doubt. The potential consequences of neglecting IRB approval can be significant, impacting your research project and possibly your career.
Latest Posts
Latest Posts
-
Stacey Lloyd 2014 Ethos Pathos Logos Answer Key
Mar 13, 2025
-
Rn Learning System Medical Surgical Immune And Infectious Practice Quiz
Mar 13, 2025
-
Lines Composed Above Tintern Abbey Summary
Mar 13, 2025
-
How Many Chapters Are In Jane Eyre
Mar 13, 2025
-
East Asia And The Pacific Rim Unit Test
Mar 13, 2025
Related Post
Thank you for visiting our website which covers about Which Of The Following Studies Would Need Irb Approval . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
