Which Product S Would Form Under The Conditions Given Below
Onlines
Mar 06, 2025 · 6 min read
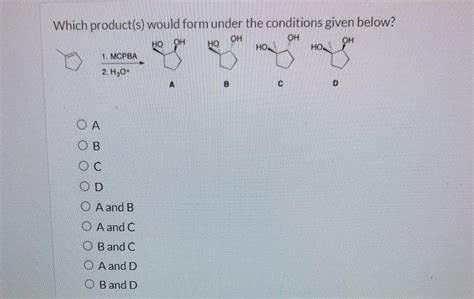
Table of Contents
Predicting Product Formation Under Specific Conditions: A Comprehensive Guide
Predicting the products formed under specific chemical conditions is a fundamental aspect of chemistry. It involves understanding reaction mechanisms, equilibrium principles, and the properties of reactants and potential products. This guide explores various scenarios and provides a framework for predicting product formation, considering factors like temperature, pressure, concentration, catalysts, and the nature of the reactants themselves.
Factors Influencing Product Formation
Several key factors significantly influence the type and quantity of products formed in a chemical reaction. Understanding these factors is crucial for accurate predictions.
1. Reactant Properties: The Foundation of Prediction
The inherent properties of the reactants form the bedrock of any prediction. This includes:
-
Chemical Nature: The functional groups present, the type of bonds (ionic, covalent, metallic), and the overall molecular structure significantly influence reactivity. For instance, alkanes are generally less reactive than alkenes due to the presence of the pi bond in alkenes. Alcohols react differently than carboxylic acids due to the different functional groups.
-
Reactivity: Some elements and compounds are inherently more reactive than others. Highly reactive metals like alkali metals react vigorously with water, while noble metals are less reactive.
-
Concentration: The concentration of reactants directly impacts the rate of reaction and, in some cases, the products formed. Higher concentrations generally lead to faster reaction rates. In equilibrium reactions, concentration shifts can favor the formation of certain products.
2. Reaction Conditions: The Modifiers
Reaction conditions act as modifiers, altering the pathway and outcome of a reaction.
-
Temperature: Temperature significantly affects the reaction rate and the equilibrium position. Increasing the temperature usually increases the reaction rate, but the effect on equilibrium depends on whether the reaction is exothermic (heat is released) or endothermic (heat is absorbed). For exothermic reactions, increasing temperature shifts the equilibrium toward reactants, while for endothermic reactions, it shifts towards products.
-
Pressure: Pressure primarily impacts reactions involving gases. Increasing pressure favors the side of the equilibrium with fewer gas molecules. High pressure can also influence reaction mechanisms, potentially leading to different product formations.
-
Catalysts: Catalysts accelerate reaction rates without being consumed themselves. They provide an alternative reaction pathway with lower activation energy, leading to faster formation of products. Importantly, catalysts can also influence which products are formed by favoring certain reaction pathways. Enzymes are biological catalysts that exhibit remarkable selectivity.
-
Solvent: The solvent can significantly impact reaction rates and product selectivity. Polar solvents generally favor reactions involving polar reactants, while nonpolar solvents favor nonpolar reactions. The solvent's ability to stabilize intermediate species can also influence product distribution.
3. Reaction Mechanisms: Unveiling the Pathway
Understanding the reaction mechanism provides a detailed insight into the stepwise process leading to product formation. This includes:
-
Stepwise vs. Concerted: Some reactions proceed through multiple steps (stepwise mechanisms), while others occur in a single step (concerted mechanisms). Stepwise mechanisms provide opportunities for different intermediates to form, potentially leading to multiple products.
-
Intermediates: Transient species formed during a reaction that are neither reactants nor products. The stability and reactivity of intermediates determine which products are eventually formed.
-
Rate-Determining Step: The slowest step in a multi-step mechanism dictates the overall reaction rate. Understanding the rate-determining step helps predict the effect of changes in reaction conditions on the product formation.
Predicting Products: Examples Across Reaction Types
Let's explore specific reaction types and how to predict product formation under different conditions.
1. Acid-Base Reactions
Acid-base reactions involve the transfer of a proton (H⁺) from an acid to a base. Predicting products is relatively straightforward:
-
Strong Acid + Strong Base: The products are always salt and water. For example, HCl (strong acid) + NaOH (strong base) → NaCl (salt) + H₂O (water).
-
Weak Acid + Strong Base: The products are the conjugate base of the weak acid and water. The extent of reaction depends on the Ka (acid dissociation constant) of the weak acid.
-
Strong Acid + Weak Base: The products are the conjugate acid of the weak base and water. The extent of reaction depends on the Kb (base dissociation constant) of the weak base.
-
Weak Acid + Weak Base: The extent of reaction and the dominant products depend on the relative strengths of the acid and base.
2. Redox Reactions
Redox reactions involve the transfer of electrons. Predicting products requires identifying the oxidizing and reducing agents and their respective half-reactions.
-
Identifying Oxidation States: Assigning oxidation states to all atoms helps determine electron transfer.
-
Balancing Half-Reactions: Balancing the number of electrons gained and lost in the oxidation and reduction half-reactions is crucial for predicting stoichiometry.
-
Combining Half-Reactions: Combining the balanced half-reactions gives the overall redox reaction and the products. For example, the reaction between Fe²⁺ and MnO₄⁻ in acidic solution leads to the formation of Fe³⁺ and Mn²⁺.
3. Precipitation Reactions
Precipitation reactions occur when two aqueous solutions react to form an insoluble solid (precipitate). Predicting products involves solubility rules:
-
Solubility Rules: Understanding solubility rules for different ionic compounds is crucial. If the combination of ions from the two reactants produces an insoluble compound based on these rules, a precipitate will form.
-
Net Ionic Equation: Writing the net ionic equation highlights the ions directly involved in the precipitation reaction.
4. Organic Reactions
Organic reactions exhibit much greater complexity due to the vast diversity of functional groups and reaction mechanisms. Predicting products requires:
-
Understanding Functional Groups: Different functional groups undergo specific reactions.
-
Reaction Mechanisms: Knowing the reaction mechanism is crucial for accurate product prediction. For example, nucleophilic substitution reactions can lead to different products depending on the substrate and nucleophile.
-
Stereochemistry: Stereochemistry plays a significant role in organic reactions, determining the spatial arrangement of atoms in the products.
-
Regioselectivity and Stereoselectivity: Understanding regioselectivity (preference for reaction at a particular site) and stereoselectivity (preference for formation of a particular stereoisomer) is vital for predicting the outcome of organic reactions.
5. Equilibrium Reactions
Equilibrium reactions are reversible, meaning the products can react to reform the reactants. Predicting product formation involves:
-
Equilibrium Constant (K): The equilibrium constant indicates the relative amounts of reactants and products at equilibrium. A large K indicates that the equilibrium favors products.
-
Le Chatelier's Principle: Le Chatelier's principle states that a change in conditions will shift the equilibrium in a way that counteracts the change. This helps predict how changes in temperature, pressure, or concentration affect product formation.
Advanced Techniques for Product Prediction
For complex systems, advanced techniques are often employed:
-
Computational Chemistry: Using computational methods, like Density Functional Theory (DFT), to model reactions and predict product formation.
-
Spectroscopy: Utilizing techniques such as NMR, IR, and Mass Spectrometry to identify and quantify products formed.
-
Kinetic Modeling: Developing kinetic models to simulate reaction pathways and predict product distribution.
Conclusion
Predicting product formation requires a thorough understanding of chemical principles, reaction mechanisms, and the interplay of various factors like reactant properties and reaction conditions. While simple reactions can be predicted relatively easily, complex reactions often require advanced techniques and computational tools. This guide provides a foundation for tackling this challenging yet crucial aspect of chemistry, offering a path towards more accurate predictions and a deeper appreciation for the intricacies of chemical transformations. By meticulously considering each influencing factor, chemists can approach prediction with increased confidence and precision, fostering innovation and advancements across numerous scientific disciplines.
Latest Posts
Latest Posts
-
Unit 6 Progress Check Mcq Ap Lang
Mar 06, 2025
-
Origin Of Species Summary By Chapter
Mar 06, 2025
-
What Are Strengths And Weaknesses Of Harrahs Gainsharing
Mar 06, 2025
-
Match Each Concept To Its Definition
Mar 06, 2025
-
Orlando Company Began Operation In December
Mar 06, 2025
Related Post
Thank you for visiting our website which covers about Which Product S Would Form Under The Conditions Given Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
