Write The Formula Formula Unit For The Following Compounds
Onlines
Mar 04, 2025 · 6 min read
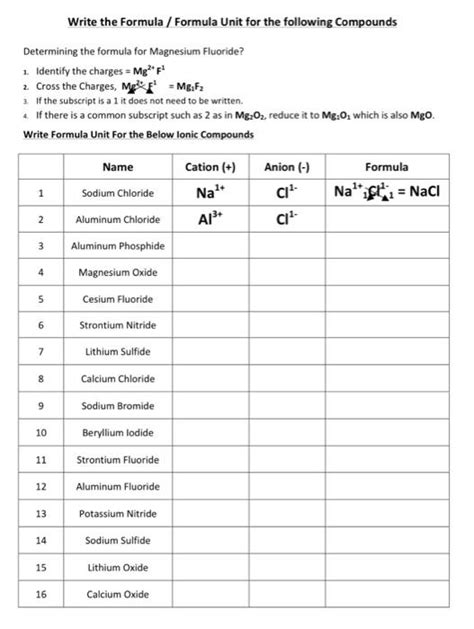
Table of Contents
Decoding Chemical Formulas: A Deep Dive into Formula Units
Understanding chemical formulas is fundamental to grasping the world of chemistry. This article will explore the concept of formula units, delve into their calculation for various compounds, and provide a comprehensive guide to mastering this essential aspect of chemical notation. We'll cover ionic compounds, covalent compounds, and the nuances involved in determining the correct formula unit for each.
What is a Formula Unit?
A formula unit is the simplest whole-number ratio of ions represented in an ionic compound. Unlike molecules, which are discrete units held together by covalent bonds, ionic compounds exist as a three-dimensional lattice of positively and negatively charged ions. The formula unit represents the repeating pattern within this lattice. For example, the formula unit for sodium chloride (NaCl) represents one sodium ion (Na⁺) and one chloride ion (Cl⁻) in the crystal lattice. It doesn't represent a single molecule, as there are no distinct NaCl molecules within the structure.
For covalent compounds, the formula unit is the same as the molecular formula, representing the actual number of atoms of each element present in a single molecule. This distinction is crucial for understanding the differences in bonding and structure between ionic and covalent substances.
Calculating Formula Units for Ionic Compounds
Ionic compounds are formed through the electrostatic attraction between positively charged cations and negatively charged anions. To determine the formula unit, we need to consider the charges of the ions involved and ensure electrical neutrality. The overall charge of the formula unit must be zero.
Step-by-step guide:
-
Identify the ions: Determine the cation and anion present in the compound. This often involves knowing the oxidation states or charges of the constituent elements.
-
Determine the charges: Assign the appropriate charges to each ion. Remember that metals typically form positive ions (cations) and non-metals typically form negative ions (anions). Consult a periodic table or a table of common ions for assistance.
-
Balance the charges: Use the criss-cross method to determine the subscripts. The absolute value of the charge of one ion becomes the subscript of the other. Simplify the ratio to the smallest whole numbers.
Examples:
-
Sodium Chloride (NaCl): Sodium (Na) has a +1 charge (Na⁺), and chlorine (Cl) has a -1 charge (Cl⁻). The charges balance perfectly, resulting in a 1:1 ratio, hence the formula unit NaCl.
-
Magnesium Oxide (MgO): Magnesium (Mg) has a +2 charge (Mg²⁺), and oxygen (O) has a -2 charge (O²⁻). The charges balance, leading to a 1:1 ratio represented by MgO.
-
Aluminum Oxide (Al₂O₃): Aluminum (Al) has a +3 charge (Al³⁺), and oxygen (O) has a -2 charge (O²⁻). Using the criss-cross method, we get Al₂O₃. The subscripts represent the ratio of aluminum ions to oxygen ions in the crystal lattice.
-
Calcium Chloride (CaCl₂): Calcium (Ca) has a +2 charge (Ca²⁺), and chlorine (Cl) has a -1 charge (Cl⁻). Applying the criss-cross method, we obtain CaCl₂.
-
Iron(III) Oxide (Fe₂O₃): Iron can exist in multiple oxidation states. In this case, it's Iron(III), having a +3 charge (Fe³⁺). Oxygen (O) has a -2 charge (O²⁻). The criss-cross method gives Fe₂O₃. The Roman numeral III indicates the oxidation state of iron.
-
Ammonium Sulfate ((NH₄)₂SO₄): This involves polyatomic ions. Ammonium (NH₄⁺) has a +1 charge, and sulfate (SO₄²⁻) has a -2 charge. The criss-cross method leads to (NH₄)₂SO₄. Note the use of parentheses to indicate that the subscript applies to the entire ammonium ion.
Calculating Formula Units for Covalent Compounds
Covalent compounds are formed by the sharing of electrons between atoms. The formula unit for a covalent compound is its molecular formula, representing the actual number of atoms of each element in a single molecule. There is no need for charge balancing as in ionic compounds. The prefixes used in naming covalent compounds directly indicate the number of atoms of each element.
Examples:
-
Carbon Dioxide (CO₂): This formula indicates one carbon atom and two oxygen atoms per molecule.
-
Water (H₂O): This formula represents two hydrogen atoms and one oxygen atom per molecule.
-
Dinitrogen Tetroxide (N₂O₄): The prefixes "di" and "tetra" indicate two nitrogen atoms and four oxygen atoms per molecule.
-
Phosphorous Pentachloride (PCl₅): The prefix "penta" indicates five chlorine atoms per molecule, combined with one phosphorus atom.
Advanced Considerations and Exceptions
While the criss-cross method is a useful tool, it's crucial to understand that it's a simplification. Some compounds may not follow this precisely. Certain elements can have variable oxidation states, leading to different formula units depending on the specific reaction conditions. Additionally, some compounds may exhibit complex structures that deviate from simple ratios.
Understanding Oxidation States: The oxidation state, or oxidation number, represents the hypothetical charge an atom would have if all bonds were completely ionic. This is especially important when dealing with transition metals which can exhibit multiple oxidation states. The oxidation state is indicated using Roman numerals in parentheses after the metal's name (e.g., Iron(II) oxide, Iron(III) oxide).
Polyatomic Ions: These are groups of atoms that carry a net charge and behave as a single unit in chemical reactions. Learning to recognize and use common polyatomic ions (like sulfate, nitrate, phosphate, ammonium) is crucial for writing correct formula units.
Hydrates: Some ionic compounds incorporate water molecules into their crystal structure. These are called hydrates, and the number of water molecules is indicated in the formula unit using a dot (·) followed by the number of water molecules. For example, copper(II) sulfate pentahydrate is written as CuSO₄·5H₂O.
Complex Ions: Some compounds involve complex ions, where a central metal ion is surrounded by ligands (molecules or ions). Determining the formula unit for these compounds requires understanding coordination chemistry principles.
Practical Applications and Importance
The ability to write correct formula units is essential for various applications in chemistry, including:
-
Stoichiometry: Calculations involving chemical reactions rely on accurately representing the amounts of reactants and products using formula units.
-
Chemical Equations: Balancing chemical equations depends on the correct formula units of the compounds involved.
-
Nomenclature: Naming chemical compounds is directly linked to the understanding of formula units and the charges of ions.
-
Qualitative and Quantitative Analysis: Accurate identification and quantification of compounds in a sample require a solid understanding of formula units.
Conclusion
Mastering the art of writing formula units is a fundamental skill for any aspiring chemist. Understanding the difference between ionic and covalent compounds, applying the criss-cross method appropriately (with awareness of its limitations), and understanding oxidation states and polyatomic ions are key to success. This article has provided a comprehensive overview, equipping you with the knowledge to confidently tackle a wide range of chemical formulas. Remember to practice frequently, and refer to periodic tables and tables of common ions as needed. With consistent effort, you will become proficient in deciphering and writing chemical formula units.
Latest Posts
Latest Posts
-
Suppose A New Technology Is Discovered Which Increases Productivity
Mar 04, 2025
-
Which Is Not Correct About A Diagnosis
Mar 04, 2025
-
A Long Walk To Water Chapter Summaries
Mar 04, 2025
-
Budgeting For Life After High School
Mar 04, 2025
-
Shallow Scratches In Sheet Metal May Be Repaired By
Mar 04, 2025
Related Post
Thank you for visiting our website which covers about Write The Formula Formula Unit For The Following Compounds . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
