Categorize The Compounds Below As Chiral Or Achiral.
Onlines
Mar 20, 2025 · 5 min read
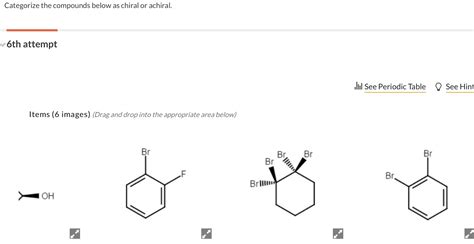
Table of Contents
Categorizing Compounds as Chiral or Achiral: A Comprehensive Guide
Chirality, a fundamental concept in organic chemistry, dictates whether a molecule is superimposable on its mirror image. Understanding chirality is crucial for various fields, including pharmaceuticals, biochemistry, and materials science. This article will delve deep into the concept of chirality, providing a clear methodology for categorizing compounds as chiral or achiral. We'll explore various examples and provide a comprehensive understanding of the underlying principles.
Understanding Chirality and Achirality
Before we start categorizing compounds, let's solidify our understanding of the key terms:
-
Chiral: A molecule is chiral if it is non-superimposable on its mirror image. Think of your hands – they are mirror images, but you can't perfectly overlay one on the other. A chiral molecule possesses a property called "handedness."
-
Achiral: A molecule is achiral if it is superimposable on its mirror image. A simple example is a sphere – its mirror image is identical to itself.
The presence of a stereocenter is often, but not always, indicative of chirality. A stereocenter is an atom with four different substituents attached. However, the presence of a stereocenter is a necessary but not sufficient condition for chirality. Some molecules with stereocenters can be achiral due to internal symmetry. We'll explore this further in the examples below.
Key Factors Determining Chirality:
- Presence of Stereocenters (asymmetric carbons): A carbon atom bonded to four different groups is a common stereocenter.
- Lack of Symmetry: Molecules with internal planes of symmetry or centers of symmetry are typically achiral.
- Conformational Isomerism: Some molecules can exist in different conformations, some of which may be chiral and others achiral. The overall chirality depends on whether the molecule can exist predominantly in a chiral conformation.
Methods for Determining Chirality
Several methods can help us determine whether a given compound is chiral or achiral:
-
Visual Inspection and Mental Manipulation: This involves drawing the molecule and its mirror image and trying to superimpose them. If they cannot be superimposed, the molecule is chiral.
-
Identifying Stereocenters: Counting the number of stereocenters provides a good starting point. However, remember that multiple stereocenters can lead to meso compounds (achiral despite having stereocenters).
-
Looking for Planes and Centers of Symmetry: If a molecule possesses a plane of symmetry (a plane that divides the molecule into two mirror-image halves) or a center of symmetry (a point in the molecule where any line drawn through it intersects an identical point on the opposite side), it is achiral.
Categorizing Examples: Chiral vs. Achiral Compounds
Let's analyze several compounds to illustrate the application of these methods.
Example 1: 2-Bromobutane
2-Bromobutane has a stereocenter (the carbon atom bonded to Br, CH3, CH2CH3, and H). Its mirror image cannot be superimposed on the original molecule. Therefore, 2-bromobutane is chiral.
Example 2: Methane (CH4)
Methane has four identical hydrogen atoms bonded to the central carbon atom. Its mirror image is identical to the original molecule; hence, methane is achiral.
Example 3: 1,2-Dibromopropane
This molecule possesses a plane of symmetry, dividing it into two identical halves. Regardless of the arrangement of the bromine atoms, it is always superimposable on its mirror image. Therefore, 1,2-Dibromopropane is achiral. Note that while it contains a stereocenter, the presence of the plane of symmetry overrides the effect of the stereocenter.
Example 4: 1,3-Dibromopropane
This molecule has no stereocenter and lacks internal symmetry. However, rotation around the C-C bonds can create conformations that are either chiral or achiral. The overall molecule would be considered achiral because of free rotation and the existence of achiral conformations.
Example 5: Tartaric Acid
Tartaric acid presents a more complex scenario. It possesses two stereocenters. One isomer, (2R,3R)-tartaric acid, is chiral. Another isomer, (2S,3S)-tartaric acid, is also chiral (it's the enantiomer of (2R,3R)-tartaric acid). However, (2R,3S)-tartaric acid, also known as meso-tartaric acid, is achiral, despite possessing two stereocenters. This is because meso-tartaric acid possesses a plane of symmetry. This highlights the importance of considering symmetry even with multiple stereocenters.
Example 6: Chlorofluoromethane (CH2ClF)
Chlorofluoromethane has a tetrahedral structure with a carbon atom bonded to four different atoms (H, Cl, F, and another H). This asymmetry makes it chiral.
Example 7: 1,1-Dichloromethane (CH2Cl2)
1,1-Dichloromethane has two chlorine atoms bonded to the same carbon atom. The molecule is symmetric and therefore achiral.
Example 8: 2,3-Dibromobutane
This molecule has two stereocenters, and depending on the configuration, it can be either chiral or achiral. The meso form (2R,3S)-2,3-dibromobutane is achiral due to its internal plane of symmetry.
Example 9: Cyclohexane
Cyclohexane in its chair conformation can be considered achiral due to its symmetry. However, some substituted cyclohexanes can be chiral if the substituents break the symmetry.
Example 10: Benzene
Benzene is a highly symmetrical molecule with a plane of symmetry through the center. Hence, it is achiral.
Advanced Considerations: Meso Compounds and Conformational Analysis
As illustrated with tartaric acid, some molecules can possess stereocenters but still be achiral. These are called meso compounds. Meso compounds have an internal plane of symmetry that renders them achiral despite having chiral centers.
Furthermore, conformational analysis is crucial for determining chirality in molecules with flexible bonds. A molecule may adopt various conformations, some chiral, and others achiral. The overall chirality depends on whether the molecule exists predominantly in a chiral conformation. If the molecule can freely interconvert between chiral and achiral conformations, it is generally considered achiral.
Conclusion
Categorizing compounds as chiral or achiral requires a careful examination of the molecule's structure, symmetry, and the presence of stereocenters. Visual inspection, identifying stereocenters and planes of symmetry, and considering conformational analysis are valuable tools for this task. Remember, the presence of a stereocenter is a necessary but not sufficient condition for chirality. The overall symmetry of the molecule ultimately dictates whether it is chiral or achiral. This understanding is paramount in many scientific disciplines, particularly in the fields of organic chemistry, pharmaceuticals, and biochemistry, where the three-dimensional structure of molecules plays a crucial role in their biological activity and properties. By mastering the principles outlined in this article, you will develop a robust understanding of chirality and its implications.
Latest Posts
Latest Posts
-
The Beaks Of Finches Lab Answers Pdf Answer Key
Mar 21, 2025
-
Conversation Between Nurse And Patient With Hypertension
Mar 21, 2025
-
Joint Fleet Maintenance Manual Volume 5
Mar 21, 2025
-
5 11 Unit Test The Power Of Language Part 1
Mar 21, 2025
-
Advanced Hardware Lab 6 3 Identify Video Ports And Connectors
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about Categorize The Compounds Below As Chiral Or Achiral. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
