Classify Each Of The Molecules Below
Onlines
Mar 22, 2025 · 5 min read
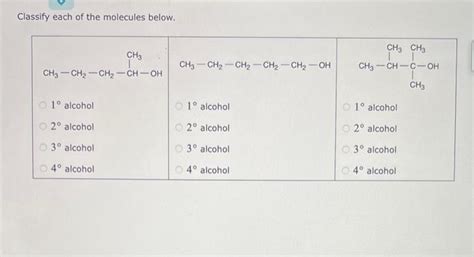
Table of Contents
Classify Each of the Following Molecules: A Comprehensive Guide
This article provides a detailed classification of various molecules, explaining the reasoning behind each classification. We'll explore different methods of categorizing molecules based on their structure, functional groups, and properties. This guide will be beneficial for students, researchers, and anyone interested in a deeper understanding of molecular classification. We will cover a wide range of examples, ensuring a thorough and comprehensive exploration of this crucial aspect of chemistry.
Note: While I cannot classify specific molecules without knowing which molecules you want me to classify, this article will give you the tools and understanding to classify molecules yourself. Please provide the molecules you'd like classified, and I will happily provide specific classifications.
Key Principles of Molecular Classification
Before diving into specific examples, let's establish the fundamental principles underlying molecular classification. Several factors contribute to how we categorize molecules:
1. Organic vs. Inorganic Molecules:
The most basic distinction is between organic and inorganic molecules.
-
Organic molecules typically contain carbon atoms bonded to hydrogen atoms, and often include oxygen, nitrogen, sulfur, and phosphorus. They are generally associated with living organisms. Examples include carbohydrates, lipids, proteins, and nucleic acids.
-
Inorganic molecules generally do not contain carbon-hydrogen bonds. They encompass a vast array of substances, including minerals, salts, and many simple compounds.
2. Functional Groups:
Functional groups are specific groups of atoms within a molecule that are responsible for its characteristic chemical reactions. Identifying these groups is crucial for classification. Some important functional groups include:
- Hydroxyl (-OH): Alcohols
- Carbonyl (C=O): Aldehydes and Ketones
- Carboxyl (-COOH): Carboxylic acids
- Amino (-NH2): Amines
- Ester (-COO-): Esters
- Ether (-O-): Ethers
- Phosphate (-PO4): Phosphates
3. Molecular Structure and Shape:
The arrangement of atoms in a molecule significantly influences its properties and therefore its classification. Considerations include:
- Isomers: Molecules with the same molecular formula but different structural arrangements (e.g., constitutional isomers, stereoisomers).
- Chain length: For hydrocarbons, the number of carbon atoms affects properties like boiling point and reactivity.
- Branching: The presence of branches in a hydrocarbon chain alters its shape and properties.
- Rings: Cyclic structures influence a molecule's rigidity and reactivity.
4. Physical and Chemical Properties:
The properties of a molecule, such as boiling point, melting point, solubility, and reactivity, are critical for classification. These properties often directly reflect the molecule's structure and functional groups.
5. Biological Activity:
For molecules found in biological systems, their biological function is a key aspect of classification. For example, enzymes are classified based on the types of reactions they catalyze.
Classifying Molecules Based on Different Criteria
Let's explore specific classification schemes based on the principles outlined above:
A. Classification Based on Functional Groups:
This is one of the most common approaches. A molecule is classified primarily based on its most significant functional group. For example:
- A molecule containing a carboxyl group (-COOH) is classified as a carboxylic acid. Examples include acetic acid (vinegar) and citric acid.
- A molecule with a hydroxyl group (-OH) is classified as an alcohol. Examples include ethanol and methanol.
- A molecule containing an amino group (-NH2) and a carboxyl group (-COOH) is classified as an amino acid. Examples include glycine, alanine, and valine. These are the building blocks of proteins.
B. Classification Based on Molecular Structure:
This classification focuses on the overall structure of the molecule. Examples include:
- Hydrocarbons: These molecules are composed solely of carbon and hydrogen atoms. They are further subdivided into alkanes (single bonds), alkenes (double bonds), alkynes (triple bonds), and aromatic hydrocarbons (containing benzene rings).
- Carbohydrates: These are classified based on their structure into monosaccharides (single sugar units), disaccharides (two sugar units), and polysaccharides (long chains of sugar units).
- Lipids: A diverse group of molecules characterized by their insolubility in water. They include fatty acids, triglycerides, phospholipids, and steroids.
- Proteins: These are polymers of amino acids, classified based on their structure (primary, secondary, tertiary, quaternary) and function (enzymes, structural proteins, etc.).
- Nucleic acids: These include DNA and RNA, which are polymers of nucleotides. They are classified based on their sugar component (deoxyribose in DNA, ribose in RNA) and their nitrogenous bases.
C. Classification Based on Chemical Properties:
This approach categorizes molecules based on their reactivity and behavior in chemical reactions. For example:
- Acids: These donate protons (H+) in solution.
- Bases: These accept protons (H+) in solution.
- Electrolytes: These substances dissociate into ions in solution, conducting electricity.
- Non-electrolytes: These do not dissociate into ions in solution.
D. Classification Based on Biological Activity:
This is particularly relevant for molecules involved in biological processes:
- Enzymes: These are biological catalysts that speed up chemical reactions.
- Hormones: These are chemical messengers that regulate various bodily functions.
- Neurotransmitters: These are chemical messengers that transmit signals between nerve cells.
- Vitamins: These are essential organic compounds needed for various metabolic processes.
- Drugs: These are substances used to treat or prevent diseases.
Advanced Considerations in Molecular Classification
The classification of molecules can become complex as the size and complexity of the molecules increase. Some advanced considerations include:
- Stereochemistry: This field deals with the three-dimensional arrangement of atoms in molecules. Stereoisomers have the same connectivity but different spatial arrangements, leading to different properties and biological activities.
- Conformational isomers: These are different spatial arrangements of a molecule that can interconvert by rotation around single bonds.
- Chirality: This refers to the property of a molecule that is not superimposable on its mirror image. Chiral molecules exhibit optical activity, rotating the plane of polarized light.
- Polymer Chemistry: This branch deals with the classification and properties of polymers, which are large molecules composed of repeating units (monomers). Polymers are often classified based on their monomer units, structure, and properties.
Conclusion
Classifying molecules is a fundamental aspect of chemistry and biochemistry. The methods described here provide a framework for understanding the various approaches used to categorize molecules based on their structure, functional groups, properties, and biological activity. While we have touched upon many aspects of molecular classification, the field remains vast and continuously evolving. As new molecules are discovered and synthesized, new classification schemes and approaches may emerge. This detailed explanation should equip you to classify various molecules effectively. Remember to consider all the factors discussed above for a comprehensive and accurate classification. Remember to provide the molecules you want classified for a personalized and precise response.
Latest Posts
Latest Posts
-
Medical Surgical Lpn Rn Assessment 1 Shiftkey Quizlet
Mar 23, 2025
-
2021 Practice Exam Mcq Ap Csp
Mar 23, 2025
-
Code Standards And Practices 4 Lesson 1
Mar 23, 2025
-
The Tenors Sing The While The Strings Perform The
Mar 23, 2025
-
Student Exploration Boyles Law And Charles Law
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Classify Each Of The Molecules Below . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
