Color By Number The Mole Answer Key
Onlines
Mar 16, 2025 · 6 min read
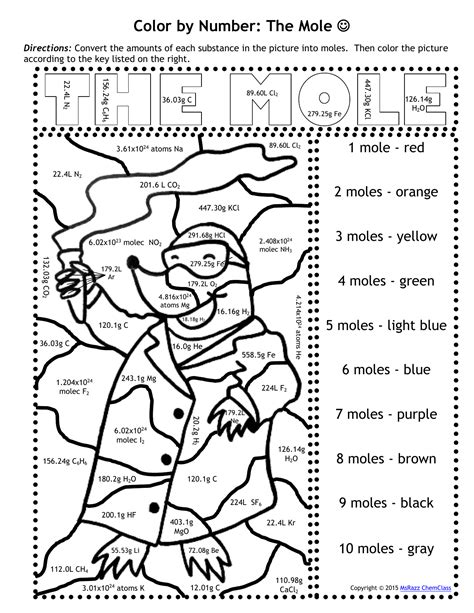
Table of Contents
Color by Number: The Mole Answer Key – A Comprehensive Guide
Are you ready to unlock the secrets of the mole? This isn't your average mole, we're talking about the fundamental unit in chemistry! Color-by-number activities can be a surprisingly effective way to learn complex concepts, and understanding the mole is no exception. This comprehensive guide will delve into the intricacies of the mole, providing you with the "answer key" to understanding this crucial concept, and showing you how to use color-by-number activities to master it.
What is a Mole? Understanding the Fundamental Unit of Chemistry
Before we dive into the color-by-number aspect, let's solidify our understanding of the mole itself. The mole (mol) is a unit of measurement used in chemistry to express amounts of a chemical substance, whether it's an element, compound, or molecule. It's analogous to using a dozen to represent 12 items; a mole represents a specific number of particles. That number is Avogadro's number, approximately 6.022 x 10²³.
Think of it this way: if you have one mole of carbon atoms, you have 6.022 x 10²³ carbon atoms. This seemingly massive number is crucial because it allows chemists to relate macroscopic properties (like mass) to microscopic properties (like the number of atoms or molecules).
The Importance of Avogadro's Number
Avogadro's number is the cornerstone of mole calculations. It's the link between the microscopic world of atoms and molecules and the macroscopic world we experience. Without Avogadro's number, we wouldn't be able to accurately determine the amount of substance in a reaction, calculate concentrations, or perform a myriad of other essential chemical calculations.
Connecting the Mole to Mass: Molar Mass
The molar mass of a substance is the mass of one mole of that substance, expressed in grams per mole (g/mol). It's essentially the atomic weight (or molecular weight) of the element or compound, but expressed in grams instead of atomic mass units (amu). For example, the molar mass of carbon (C) is approximately 12.01 g/mol. This means that one mole of carbon atoms has a mass of 12.01 grams.
Calculating Molar Mass
Calculating molar mass involves summing the atomic masses of all the atoms in a molecule. For instance, to find the molar mass of water (H₂O), we add the atomic mass of two hydrogen atoms (1.01 g/mol each) and one oxygen atom (16.00 g/mol):
2(1.01 g/mol) + 16.00 g/mol = 18.02 g/mol
Therefore, the molar mass of water is 18.02 g/mol.
Color-by-Number Activities: Visualizing the Mole
Now, let's get to the fun part! Color-by-number worksheets can be a fantastic way to reinforce the concepts of the mole and molar mass. These activities can be designed to involve:
-
Converting moles to grams: A worksheet might ask you to calculate the mass of a given number of moles of a substance, requiring you to use the molar mass. The answer would correspond to a specific color on the color-by-number image.
-
Converting grams to moles: Conversely, a worksheet could present you with a mass of a substance and ask you to calculate the number of moles. Again, the answer determines the color.
-
Stoichiometry problems: More advanced worksheets could incorporate stoichiometry problems, requiring you to use balanced chemical equations to calculate the moles of reactants or products. The solution to each step would unlock a different color in the image.
-
Avogadro's number applications: Problems could directly involve Avogadro's number, asking to calculate the number of atoms or molecules in a given number of moles.
Example Color-by-Number Problem:
Let's say you have a color-by-number worksheet depicting a periodic table element, such as oxygen. One section might ask:
"Calculate the mass of 2.5 moles of oxygen (O₂). Use the molar mass of O₂ (32 g/mol). Color this section blue if your answer is 80g."
The solution: 2.5 moles * 32 g/mol = 80 g. Therefore, you color that section blue.
Advanced Applications: Beyond the Basics
Once you've mastered the fundamental color-by-number activities, you can move on to more advanced applications of the mole concept:
-
Empirical and Molecular Formulas: Worksheets could incorporate problems that require you to determine the empirical or molecular formula of a compound, given its mass composition and molar mass.
-
Solution Chemistry: Problems involving molarity, molality, and other concentration units can also be incorporated into color-by-number exercises.
-
Gas Laws: The ideal gas law (PV = nRT) offers opportunities to connect the mole concept to gas volume, pressure, and temperature. Color-by-number problems could involve calculating the volume of a gas given its number of moles under specific conditions.
-
Titration Calculations: Stoichiometry problems related to titrations can be used to create complex and engaging color-by-number challenges.
Creating Your Own Color-by-Number Worksheets: A Step-by-Step Guide
You can easily create your own customized color-by-number worksheets to reinforce the mole concept. Here’s a step-by-step guide:
-
Choose an image: Select a simple, line-art image that's easy to divide into sections.
-
Divide the image: Divide the image into numbered sections, each corresponding to a problem.
-
Create problems: Develop problems related to the mole concept, with the answer to each problem corresponding to a specific color. Make sure the difficulty aligns with the learning level.
-
Create a color key: Create a key that matches each problem answer with a color.
-
Assemble the worksheet: Combine the image, numbered sections, problems, and color key into a single, printable worksheet.
Benefits of Using Color-by-Number Activities
Color-by-number activities offer several benefits for learning chemistry:
-
Engaging and Fun: They transform a potentially dry topic into a fun and engaging activity.
-
Visual Learning: They cater to visual learners by providing a visual representation of the concepts.
-
Reinforcement: They reinforce learning by connecting abstract concepts to a concrete activity.
-
Self-Paced Learning: Learners can work at their own pace, focusing on areas where they need more practice.
-
Differentiated Instruction: Worksheets can be easily differentiated to suit various learning levels.
Conclusion: Unlocking the Mole, One Color at a Time
The mole is a fundamental concept in chemistry, and mastering it is crucial for success in the field. Color-by-number activities provide a unique and effective way to learn and reinforce this important concept. By combining visual learning with problem-solving, these activities make learning chemistry more engaging and accessible, allowing students to unlock the secrets of the mole, one color at a time. Remember to practice consistently, and don't be afraid to experiment with different types of problems to challenge yourself and solidify your understanding. Happy coloring!
Latest Posts
Latest Posts
-
An Electrical Motor Provides 0 50 W Of Mechanical Power
Mar 17, 2025
-
Studying Marketing Should Help You To Blank
Mar 17, 2025
-
Shaping Clay On A Rapidly Turning Wheel Is Called
Mar 17, 2025
-
During The International Coronavirus Pandemic Many People
Mar 17, 2025
-
Heart Failure With Afib Hesi Case Study
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Color By Number The Mole Answer Key . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
