Data Table 1 Diffusion Of Kmno4
Onlines
Mar 28, 2025 · 5 min read
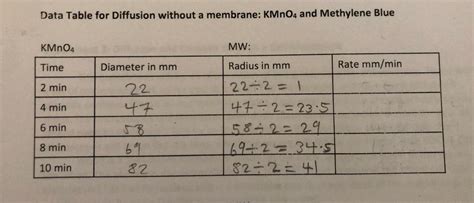
Table of Contents
Data Table 1: Diffusion of KMnO4 – A Comprehensive Analysis
The diffusion of potassium permanganate (KMnO4) is a classic experiment demonstrating the principles of diffusion and concentration gradients. This article will delve deep into the analysis of a hypothetical Data Table 1 concerning this experiment, exploring the underlying scientific principles, potential sources of error, and how to improve experimental design for more accurate and reliable results. We will also discuss the significance of this experiment in various scientific fields.
Understanding the Experiment: Diffusion of KMnO4
Potassium permanganate (KMnO4) is a vibrant purple crystalline compound highly soluble in water. When a crystal of KMnO4 is placed in water, it dissolves and its ions (K+ and MnO4-) begin to diffuse throughout the solution. Diffusion is the net movement of particles from a region of higher concentration to a region of lower concentration. This process continues until the concentration of KMnO4 is uniform throughout the solution, resulting in a diluted purple color. The rate of diffusion is influenced by several factors, including temperature, the size and shape of the container, the concentration gradient, and the properties of the solvent.
Hypothetical Data Table 1: KMnO4 Diffusion
Let's assume our hypothetical Data Table 1 records the following information over a specific time interval (e.g., in minutes):
| Time (minutes) | Distance of Diffusion Front (cm) | Concentration at Diffusion Front (arbitrary units) | Observations |
|---|---|---|---|
| 0 | 0 | 100 | Crystal of KMnO4 placed in water |
| 5 | 1 | 80 | Purple color spreading slowly |
| 10 | 2 | 60 | Diffusion noticeably faster |
| 15 | 3 | 40 | Color becoming more diluted |
| 20 | 4 | 20 | Diffusion slowing down |
| 25 | 4.5 | 10 | Color approaching uniformity |
| 30 | 5 | 5 | Solution nearly uniformly colored |
| 35 | 5 | 5 | Uniform purple color established |
Note: The concentration values in this table are arbitrary units, as the precise measurement requires spectrophotometric analysis. The "Observations" column is crucial for recording qualitative data, adding valuable context to the quantitative measurements.
Analyzing Data Table 1: Key Observations and Interpretations
Several key observations can be drawn from this hypothetical data:
1. Initial Rapid Diffusion:
During the initial stages (0-10 minutes), the diffusion front advances rapidly. This is expected because the concentration gradient is steepest at the beginning, leading to a high driving force for diffusion.
2. Decreasing Rate of Diffusion:
As time progresses, the rate of diffusion slows down. This is because the concentration gradient decreases as the KMnO4 spreads out, reducing the driving force for diffusion.
3. Concentration Gradient:
The data clearly shows a decreasing concentration of KMnO4 at the diffusion front as time elapses. This directly reflects the spreading and dilution of the solute.
4. Equilibrium:
The data suggests that the system eventually reaches equilibrium (around 30-35 minutes) where the concentration of KMnO4 is approximately uniform throughout the solution. At equilibrium, the net movement of KMnO4 molecules ceases.
Factors Affecting Diffusion: A Deeper Dive
Several factors influence the rate of diffusion, and deviations from expected results in Data Table 1 could be attributed to these:
1. Temperature:
Higher temperatures increase the kinetic energy of the KMnO4 ions and water molecules, leading to faster diffusion. Conversely, lower temperatures would slow down the process.
2. Concentration:
A higher initial concentration of KMnO4 would result in faster initial diffusion due to a steeper concentration gradient.
3. Solvent Properties:
The viscosity of the solvent affects diffusion; a less viscous solvent facilitates faster diffusion.
4. Size and Shape of the Container:
The geometry of the container influences the diffusion pathways. A larger container or a container with complex geometry would increase the diffusion time.
5. Stirring/Agitation:
The absence of stirring in this experiment allows for purely diffusive movement. Stirring or agitation would significantly accelerate the mixing process.
Sources of Error and Experimental Improvements
Several sources of error can affect the accuracy and reliability of Data Table 1:
1. Subjective Measurement of Diffusion Front:
The determination of the diffusion front might be subjective, leading to variations in measurements. Using a more precise method, such as image analysis software, could improve accuracy.
2. Inconsistent Temperature:
Fluctuations in temperature throughout the experiment could influence the rate of diffusion. Maintaining a constant temperature is crucial.
3. Impurities in KMnO4 or Water:
Impurities in the KMnO4 crystal or the water could affect the diffusion process. Using high-purity chemicals and distilled water is essential.
4. Evaporation:
Evaporation of water could potentially alter the concentration of KMnO4 over time. The experiment should be conducted in a closed container to minimize evaporation.
5. Incomplete Mixing:
If the solution isn't thoroughly mixed before the start of the experiment, the initial concentration might not be uniform, affecting the results.
To improve the experiment, consider:
- Using a more precise method for measuring the diffusion front.
- Controlling the temperature using a thermostat.
- Using high-purity chemicals and distilled water.
- Conducting the experiment in a sealed container to minimize evaporation.
- Repeating the experiment multiple times to obtain statistically significant results.
- Employing spectrophotometry to quantify the KMnO4 concentration precisely.
Applications and Significance
The diffusion of KMnO4 is not merely a classroom demonstration; it has practical applications in several fields:
1. Environmental Science:
Understanding diffusion is critical in environmental studies, particularly in assessing the spread of pollutants in water bodies and soil.
2. Biology and Medicine:
Diffusion plays a crucial role in biological processes like nutrient transport across cell membranes and oxygen transport in the bloodstream.
3. Chemical Engineering:
Diffusion principles are crucial in chemical engineering processes, such as mixing, separation, and reaction engineering.
4. Material Science:
Diffusion is involved in various material science processes, including doping of semiconductors and the synthesis of new materials.
Conclusion
The diffusion of KMnO4, as represented in our hypothetical Data Table 1, offers a valuable insight into the fundamental principles of diffusion. While the experiment is seemingly simple, its analysis reveals a complex interplay of factors that influence the diffusion process. By understanding these factors and implementing improvements to the experimental design, we can obtain more precise and reliable data, enhancing our comprehension of this fundamental physical phenomenon with significant implications across various scientific disciplines. The careful consideration of sources of error, coupled with the use of more precise measurement techniques, will contribute to more robust and meaningful experimental results. The experiment remains an invaluable tool for teaching and research, continuing to illuminate the intricate mechanisms governing molecular movement and its consequences.
Latest Posts
Latest Posts
-
Aetna Claim Benefit Specialist Virtual Job Tryout Answers
Mar 31, 2025
-
Should We Unplug The Drip Brewer Before Partially Disassembling It
Mar 31, 2025
-
Domain 3 Lesson 2 Fill In The Blanks
Mar 31, 2025
-
The Outsiders Book Chapter 4 Summary
Mar 31, 2025
-
2 2 Tangent Lines And The Derivative Homework Answer Key
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about Data Table 1 Diffusion Of Kmno4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
