Draw An Alkyl Halide That Would Undergo An Sn2 Reaction
Onlines
Mar 22, 2025 · 6 min read
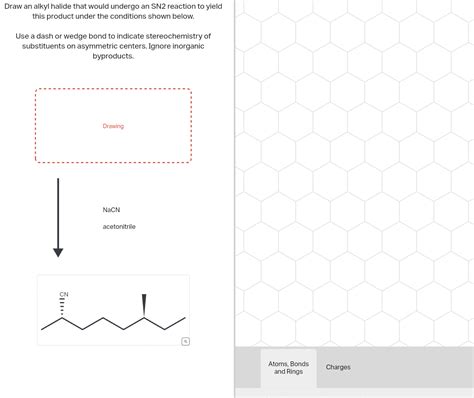
Table of Contents
Drawing Alkyl Halides Favoring SN2 Reactions: A Comprehensive Guide
The SN2 reaction, a cornerstone of organic chemistry, involves a nucleophilic attack on an alkyl halide, resulting in a substitution. Understanding which alkyl halides readily undergo SN2 reactions is crucial for synthetic organic chemistry. This comprehensive guide delves into the factors influencing SN2 reactivity and provides a detailed approach to drawing suitable alkyl halide structures. We'll explore steric hindrance, leaving group ability, solvent effects, and nucleophile strength, ultimately equipping you to predict and design SN2 reactions effectively.
Understanding the SN2 Mechanism
Before diving into drawing suitable alkyl halides, let's revisit the SN2 mechanism. This is a concerted, bimolecular process, meaning the bond breaking and bond formation occur simultaneously in a single transition state. The nucleophile attacks the carbon atom bearing the halogen from the backside, leading to inversion of configuration at the stereocenter (if present). This backside attack is key and highlights the importance of steric factors.
Factors Influencing SN2 Reactivity
Several factors significantly influence the rate and feasibility of an SN2 reaction:
-
Steric Hindrance: This is arguably the most crucial factor. Bulky substituents around the carbon atom bearing the leaving group (the alpha carbon) hinder the approach of the nucleophile. Methyl halides (CH3X) react fastest, followed by primary (1°) alkyl halides, then secondary (2°) alkyl halides. Tertiary (3°) alkyl halides rarely undergo SN2 reactions due to significant steric hindrance.
-
Leaving Group Ability: A good leaving group is a weak base and readily departs with its electron pair. Common good leaving groups include halides (I⁻ > Br⁻ > Cl⁻ > F⁻), tosylates (OTs⁻), and mesylates (OMs⁻). The weaker the conjugate acid of the leaving group, the better it is.
-
Nucleophile Strength: A strong nucleophile is essential for SN2 reactions. Strong nucleophiles possess a high electron density and readily donate an electron pair. Examples include: HO⁻, RO⁻ (alkoxides), RS⁻ (thiolates), CN⁻, and I⁻.
-
Solvent Effects: Polar aprotic solvents, like DMSO (dimethyl sulfoxide), DMF (dimethylformamide), and acetone, are preferred for SN2 reactions. These solvents solvate the cation but not the nucleophile, keeping the nucleophile highly reactive. Polar protic solvents, like water and alcohols, can solvate both the nucleophile and cation, reducing the nucleophile's effectiveness.
Drawing Alkyl Halides for SN2 Reactions
Now, let's apply this knowledge to drawing alkyl halides that are suitable substrates for SN2 reactions:
1. Methyl Halides
Methyl halides are the ideal substrates for SN2 reactions due to the absence of steric hindrance.
Example: CH₃Br (methyl bromide)
H
|
H - C - Br
|
H
2. Primary Alkyl Halides
Primary alkyl halides have only one alkyl group attached to the alpha carbon. They undergo SN2 reactions readily but at a slower rate than methyl halides due to slightly increased steric hindrance.
Example: CH₃CH₂Cl (ethyl chloride)
CH3
|
H - C - Cl
|
H
Another Example: 1-bromobutane
CH3-CH2-CH2-CH2-Br
This is a primary alkyl halide because the carbon atom bonded to the bromine (the leaving group) is only bonded to one other carbon atom.
3. Secondary Alkyl Halides
Secondary alkyl halides have two alkyl groups attached to the alpha carbon. These are less reactive towards SN2 reactions than primary alkyl halides due to increased steric hindrance. They often compete with SN1 reactions, particularly in polar protic solvents.
Example: 2-bromopropane
CH3
|
CH3 - C - Br
|
H
Note: While SN2 reactions can occur with secondary alkyl halides, the reaction rate is significantly slower, and the conditions must be carefully controlled to favor SN2 over SN1. The choice of nucleophile, solvent and reaction temperature plays a crucial role.
4. Tertiary Alkyl Halides
Tertiary alkyl halides have three alkyl groups attached to the alpha carbon. The extreme steric hindrance completely prevents SN2 reactions. They predominantly undergo SN1 reactions or elimination reactions (E1 or E2).
Example: (CH₃)₃CCl (tert-butyl chloride) - This will NOT undergo an SN2 reaction.
CH3
|
CH3 - C - Cl
|
CH3
Optimizing SN2 Reactions: Choosing the Right Nucleophile and Solvent
Choosing the appropriate nucleophile and solvent is crucial for successful SN2 reactions.
Strong Nucleophiles:
As previously mentioned, strong nucleophiles are key. The nucleophilicity of anionic species generally increases down the periodic table (due to increased size and polarizability) and to the left across a period (due to decreased electronegativity).
Polar Aprotic Solvents:
These solvents are preferred because they solvate the cation but leave the nucleophile less solvated, thereby increasing its reactivity.
Examples of Alkyl Halides and Reaction Conditions Favoring SN2:
Let's illustrate with specific examples:
1. Reaction: CH₃Br + NaI → CH₃I + NaBr
- Alkyl halide: Methyl bromide (CH₃Br) – a primary halide and excellent substrate for SN2
- Nucleophile: Iodide ion (I⁻) – a strong nucleophile
- Solvent: Acetone – a polar aprotic solvent, ideal for SN2
2. Reaction: CH₃CH₂Br + KCN → CH₃CH₂CN + KBr
- Alkyl halide: Ethyl bromide (CH₃CH₂Br) – a primary halide
- Nucleophile: Cyanide ion (CN⁻) – a strong nucleophile
- Solvent: DMF – a polar aprotic solvent suitable for SN2
3. Reaction: CH₃CH₂CH₂Br + NaOH → CH₃CH₂CH₂OH + NaBr
- Alkyl halide: Propyl bromide (CH₃CH₂CH₂Br) – a primary halide
- Nucleophile: Hydroxide ion (OH⁻) – a strong nucleophile, although relatively weaker than I⁻ or CN⁻.
- Solvent: Ethanol (a protic solvent, less ideal but still possible) - In this example, we might need a higher concentration of hydroxide ions or elevated temperature to overcome the solvation of the hydroxide ions by the solvent.
Note: Even with primary halides, the reaction conditions influence the outcome. Higher concentrations of nucleophiles, higher temperatures and suitable solvent selection can improve the yield of the SN2 product even in less than ideal conditions.
Advanced Considerations: Steric Effects in More Complex Molecules
When dealing with more complex molecules, the subtle steric effects become even more critical. The presence of bulky groups even in seemingly primary halides can significantly impede the SN2 reaction. Consider these examples:
-
Neopentyl bromide: (CH₃)₃CCH₂Br – While it's technically a primary halide, the neopentyl group causes significant steric hindrance, severely hindering SN2 reactions. The SN2 pathway is greatly disfavored, and other reactions such as elimination will be more prevalent.
-
Sterically hindered primary halides: Branched alkyl chains close to the alpha-carbon impede access for the nucleophile.
Conclusion: Designing Your SN2 Reaction
Designing a successful SN2 reaction hinges on a careful consideration of all the factors: steric hindrance, leaving group ability, nucleophile strength, and solvent effects. By selecting the appropriate alkyl halide, nucleophile, and solvent, you can optimize the reaction conditions to favor the SN2 pathway and achieve a high yield of the desired substitution product. Remember that methyl and primary alkyl halides are your best bets, but secondary halides can also participate, provided the reaction conditions are meticulously chosen. Tertiary halides are essentially incompatible with this mechanism. Understanding these principles allows for the strategic design and successful execution of numerous SN2 reactions in organic synthesis.
Latest Posts
Latest Posts
-
Select All Of The Following Which Describe The Modern Influence
Mar 23, 2025
-
Baroque Trumpets Were Still Natural Meaning They
Mar 23, 2025
-
A Model For Circuits Part 2 Potential Difference
Mar 23, 2025
-
Complete This Statement Food Service Gloves
Mar 23, 2025
-
Which Fossil Find Is Represented In This Graphic
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Draw An Alkyl Halide That Would Undergo An Sn2 Reaction . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
