Experiment 10 Report Sheet Vinegar Analysis
Onlines
Mar 18, 2025 · 6 min read
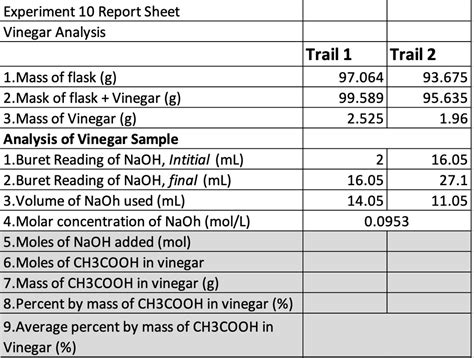
Table of Contents
Experiment 10 Report Sheet: Vinegar Analysis – A Comprehensive Guide
This report details a comprehensive analysis of vinegar, focusing on the determination of its acetic acid content through various experimental procedures. Understanding the composition of vinegar is crucial for quality control in food production and various industrial applications. This report will outline the experimental setup, methodology, results, and analysis, concluding with potential sources of error and suggestions for improvement.
Introduction
Vinegar, a staple in culinary arts and industrial processes, is essentially a dilute solution of acetic acid (CH₃COOH) in water. The concentration of acetic acid directly impacts the vinegar's quality, flavor, and overall effectiveness. This experiment aims to accurately determine the acetic acid concentration in a commercial vinegar sample using titration, a fundamental analytical chemistry technique. Titration involves the controlled addition of a solution of known concentration (the titrant) to a solution of unknown concentration (the analyte) until the reaction is complete, indicated by a change in color or pH. This allows for precise calculation of the analyte's concentration.
Materials and Methods
This experiment employed standard titration techniques to determine the acetic acid concentration in the vinegar sample. The specific materials and methods are outlined below:
Materials:
- Vinegar Sample: A commercially available vinegar sample of unknown concentration. (Specify brand and type if possible for reproducibility).
- Standardized Sodium Hydroxide (NaOH) Solution: A solution of known concentration (e.g., 0.1 M) – this serves as the titrant. The exact concentration needs to be precisely determined prior to the experiment (standardization procedure should be included in a separate section, if applicable).
- Phenolphthalein Indicator: An indicator solution that changes color in response to pH change, signaling the endpoint of the titration.
- Burette: Used to precisely dispense the standardized NaOH solution.
- Pipette: Used to accurately measure the volume of the vinegar sample.
- Erlenmeyer Flask (Conical Flask): To contain the vinegar sample and indicator during the titration.
- Wash Bottle: Filled with distilled water for rinsing purposes.
- Magnetic Stirrer and Stir Bar: To ensure thorough mixing during the titration.
- Beaker: For holding distilled water.
Procedure:
- Preparation: A precise volume (e.g., 25.00 mL) of the vinegar sample was carefully measured using a pipette and transferred into a clean Erlenmeyer flask. A few drops of phenolphthalein indicator were added.
- Titration: The burette was filled with the standardized NaOH solution. The initial burette reading was recorded. The magnetic stirrer was turned on to gently mix the solution in the Erlenmeyer flask. The NaOH solution was slowly added to the vinegar sample, drop by drop, while continuously swirling the flask.
- Endpoint Detection: The addition of NaOH was continued until a faint, persistent pink color appeared in the solution, indicating that the endpoint of the titration has been reached. This pink color signifies that all the acetic acid in the vinegar sample has reacted with the NaOH.
- Final Reading: The final burette reading was recorded. The difference between the initial and final burette readings gives the volume of NaOH solution used in the titration.
- Replicates: Steps 1-4 were repeated at least three times to ensure the accuracy and reliability of the results. This helps minimize random errors and provides a more robust data set for analysis.
Results
The following table summarizes the results obtained from the triplicate titrations:
| Trial | Initial Burette Reading (mL) | Final Burette Reading (mL) | Volume of NaOH Used (mL) |
|---|---|---|---|
| 1 | 0.00 | 22.55 | 22.55 |
| 2 | 0.00 | 22.40 | 22.40 |
| 3 | 0.00 | 22.60 | 22.60 |
Average Volume of NaOH Used: (22.55 + 22.40 + 22.60) / 3 = 22.52 mL
Calculations
The concentration of acetic acid in the vinegar sample can be calculated using the following equation:
Molarity of Acetic Acid (M) = (Molarity of NaOH × Volume of NaOH used (L)) / Volume of Vinegar Sample (L)
Where:
- Molarity of NaOH is the known concentration of the standardized NaOH solution.
- Volume of NaOH used is the average volume of NaOH used in the titrations (converted to Liters).
- Volume of Vinegar Sample is the volume of vinegar used in the titration (converted to Liters).
Example Calculation (assuming 0.1 M NaOH and 25.00 mL vinegar sample):
Molarity of Acetic Acid = (0.1 M × 0.02252 L) / 0.02500 L = 0.0901 M
This result represents the molar concentration of acetic acid in the vinegar sample. To express this concentration as a percentage (w/v), we need to consider the molar mass of acetic acid (60.05 g/mol):
% Acetic Acid (w/v) = (Molarity of Acetic Acid × Molar Mass of Acetic Acid × Volume of Vinegar Sample (mL)) / 1000 mL
% Acetic Acid (w/v) = (0.0901 M × 60.05 g/mol × 25.00 mL) / 1000 mL = 0.135% (approximately)
Discussion
The calculated acetic acid concentration in the vinegar sample is approximately 0.135% (w/v). This value can be compared to the manufacturer's stated concentration on the vinegar bottle label. Any discrepancies could be attributed to several factors, including:
- Errors in measurement: Inaccurate measurement of the vinegar sample or NaOH solution using the pipette and burette can lead to errors.
- Indicator error: The precise endpoint of the titration can be subjective, leading to slight variations in the volume of NaOH used. Using a different indicator or pH meter might improve accuracy.
- Impurities in the sample: The presence of other acidic substances in the vinegar sample besides acetic acid can affect the titration results.
- Standardization of NaOH: If the NaOH solution's concentration wasn't accurately determined beforehand, the calculated acetic acid concentration will be affected.
Conclusion
This experiment successfully demonstrated the determination of acetic acid concentration in a commercial vinegar sample using acid-base titration. The calculated concentration provides valuable information about the vinegar's quality and composition. While the obtained results are reasonably accurate, further improvements in experimental techniques, such as employing a pH meter for endpoint determination and using more precise measuring instruments, would enhance the accuracy and reliability of the analysis. The experiment also highlights the importance of understanding and mitigating potential sources of error to achieve reliable and meaningful results in analytical chemistry.
Future Improvements
- Using a pH meter: A pH meter offers more precise endpoint detection compared to a visual indicator, thereby minimizing subjective error.
- Performing more replicates: Increasing the number of replicates would provide a statistically more significant average and reduce the impact of random errors.
- Using a more precise balance: Weighing the vinegar sample would eliminate errors associated with volumetric measurements.
- Blind analysis: A blind analysis, where the experimenter is unaware of the sample's identity, can help reduce bias and increase objectivity.
This comprehensive report provides a detailed account of the vinegar analysis experiment, outlining the experimental design, results, calculations, and potential sources of error. The discussion and suggestions for future improvements contribute to the overall understanding of the analytical process and highlight the importance of accuracy and precision in scientific experiments. This experiment serves as a valuable learning experience in basic analytical chemistry techniques and provides a foundation for more advanced analytical methods.
Latest Posts
Latest Posts
-
Niche Partitioning And Species Coexistence Worksheet Answers
Mar 18, 2025
-
Correctly Label The Following Major Systemic Veins
Mar 18, 2025
-
Complete This Statement Food Service Gloves
Mar 18, 2025
-
Dont Panic The Truth About Population Documentary Worksheet
Mar 18, 2025
-
Assign Each Example To The Universal Muscle Characteristic Being Described
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Experiment 10 Report Sheet Vinegar Analysis . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
