Given The Planar Trisubstituted Cyclohexane Fill In The Missing Substituents
Onlines
Mar 28, 2025 · 5 min read
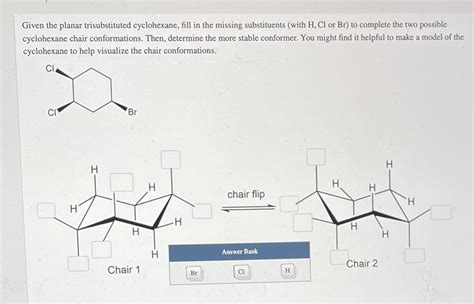
Table of Contents
- Given The Planar Trisubstituted Cyclohexane Fill In The Missing Substituents
- Table of Contents
- Given a Planar Trisubstituted Cyclohexane: Filling in the Missing Substituents
- Understanding the Limitations of Planar Representation
- Analyzing a Planar Trisubstituted Cyclohexane
- Applying the CIP Rules (Cahn-Ingold-Prelog)
- Considering Chair Conformations for Accuracy
- Practical Example and Step-by-Step Solution
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Given a Planar Trisubstituted Cyclohexane: Filling in the Missing Substituents
Understanding the stereochemistry of cyclohexane derivatives is crucial in organic chemistry. Cyclohexane, a six-membered ring, isn't actually planar; it adopts a chair conformation to minimize steric strain. However, for simplification, especially in introductory organic chemistry, we sometimes encounter problems depicting cyclohexane rings as planar triangles. This article explores the challenge of determining the missing substituents in a planar trisubstituted cyclohexane, highlighting the limitations of this representation and providing a methodical approach to solving such problems.
Understanding the Limitations of Planar Representation
Before diving into problem-solving, it's vital to acknowledge the inherent limitations of portraying cyclohexane as a planar structure. The actual chair conformation is significantly more complex, featuring axial and equatorial positions for substituents which drastically affect their steric interactions. A planar representation obscures this crucial 3D information. Therefore, any conclusions drawn from a planar depiction should be treated with caution and verified using a more realistic model.
Analyzing a Planar Trisubstituted Cyclohexane
Let's consider a hypothetical scenario: you are given a planar representation of a trisubstituted cyclohexane with two substituents already assigned. For example, let's say we have carbon 1 with a methyl group (CH3) and carbon 3 with a bromine atom (Br). The task is to determine the configuration of the substituent at carbon 5.
CH3
|
1---2---3
| |
| | Br
5---4---
|
?
Applying the CIP Rules (Cahn-Ingold-Prelog)
The CIP rules are the cornerstone of assigning absolute configuration (R or S) to chiral centers. While this planar representation isn't ideal, we can still tentatively apply the CIP rules to predict the stereochemistry of the missing substituent. Remember, the accuracy of this prediction depends heavily on the assumption of a planar structure.
1. Prioritize the Substituents: We must prioritize the three substituents attached to carbon 5 according to their atomic number. Let’s assume the missing substituent is “X”. We would compare the atomic numbers of the atoms directly bonded to carbon 5. This comparison determines the priority order. Typically, the highest atomic number receives the highest priority (1), the next highest receives priority 2, and so on.
2. Determine the Configuration: Once prioritized, we can tentatively visualize the arrangement in the plane. We'll consider two possible configurations:
-
Scenario A (X above the plane): If X is positioned above the plane (assuming the plane of the ring is the reference plane), we would draw the arrangement and follow the CIP rules' sequence of priorities (1, 2, 3). If the sequence is clockwise, it's tentatively labeled as (R); if counter-clockwise, it's tentatively (S).
-
Scenario B (X below the plane): If X is positioned below the plane, we would similarly draw it, assign priority according to the CIP rules, and determine its tentative (R) or (S) configuration.
Important Note: This approach only offers a tentative prediction based on an inaccurate planar model. The actual stereochemistry in the chair conformation could differ.
Considering Chair Conformations for Accuracy
The planar representation is a significant simplification. To determine the true configuration, we must consider the chair conformations of cyclohexane. Each substituent can exist in either an axial or equatorial position. The most stable conformation generally positions the largest substituents in equatorial positions to minimize steric hindrance (1,3-diaxial interactions).
-
Draw both chair conformations: Draw both chair conformations of the cyclohexane with the known substituents (CH3 and Br) in both axial and equatorial positions.
-
Add the third substituent: Add the possible substituents "X" in all possible combinations (axial/equatorial) in both chair conformations.
-
Evaluate steric strain: Analyze the steric strain in each conformation. The conformation with the least steric strain is generally the most stable and represents the preferred configuration. This is where the true stereochemistry is revealed.
-
Assign (R) or (S): Based on the most stable conformation, assign the (R) or (S) configuration using the CIP rules.
Practical Example and Step-by-Step Solution
Let's work through a specific example. Let’s assume we have a planar trisubstituted cyclohexane with a methyl group at carbon 1, a bromine at carbon 3, and we need to find the substituent at carbon 5. Let's assume this unknown substituent, X, is an ethyl group.
1. Planar Representation:
CH3
|
1---2---3
| |
| | Br
5---4---
|
Et
2. Tentative Planar CIP Configuration (Incorrect): Using a planar approach, we attempt to determine the configuration at C5. This step is primarily for illustrative purposes and to highlight its limitations.
3. Chair Conformations: Now we'll analyze chair conformations. Remember, the most stable conformations place the largest groups equatorially.
4. Evaluating Steric Interactions: For each conformation, consider 1,3-diaxial interactions. These are steric clashes between axial substituents and hydrogen atoms on carbons three positions away. A conformation with fewer 1,3-diaxial interactions is more stable.
5. Determining the Most Stable Conformation: After analyzing both chair conformations, you can select the more stable one. The conformation with the minimum steric interactions will correspond to the most stable form.
6. Final (R)/(S) Assignment: Based on the substituent arrangement in the most stable conformation, use the CIP rules to assign the correct (R) or (S) configuration to the chiral carbon. This gives the accurate stereochemical designation.
Conclusion
While a planar representation of a trisubstituted cyclohexane can offer a simplified starting point, it is insufficient for accurately determining the configuration of the missing substituents. The chair conformations must be considered to account for the three-dimensional nature of the molecule and the crucial role of steric effects. This approach guarantees a more reliable assignment of stereochemistry (R or S) for the molecule and illustrates the importance of utilizing appropriate conformational analysis techniques in stereochemistry. Always remember that the planar representation is a tool for initial visualization, and accurate stereochemical determination requires analyzing the chair conformations and considering steric interactions.
Latest Posts
Latest Posts
-
A Large Hq Staff Is Generally Better For Decision Making
Apr 01, 2025
-
What Tool Detangles And Styles Wigs Hairpieces And Hair Additions
Apr 01, 2025
-
The Graphs Below Depict Hypothesized Population Dynamics
Apr 01, 2025
-
Behavior Intervention Plans Are Used In Clinical Settings Only
Apr 01, 2025
-
How Does A Program Office Team Request A Volt
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Given The Planar Trisubstituted Cyclohexane Fill In The Missing Substituents . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
