Here Are Sketches Of Four Electron Orbitals
Onlines
Apr 01, 2025 · 7 min read
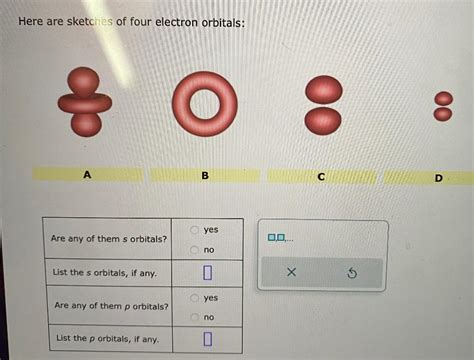
Table of Contents
Here Are Sketches of Four Electron Orbitals: A Deep Dive into Atomic Structure
Understanding the structure of atoms is fundamental to comprehending chemistry and physics. At the heart of this understanding lies the concept of electron orbitals – regions of space around an atom's nucleus where there's a high probability of finding an electron. While we can't pinpoint an electron's exact location due to the Heisenberg Uncertainty Principle, orbitals provide a probabilistic model that accurately describes electron behavior. This article will delve into the sketches of four common electron orbitals: s, p<sub>x</sub>, p<sub>y</sub>, and p<sub>z</sub>, exploring their shapes, orientations, and significance in chemical bonding and atomic properties.
The Significance of Electron Orbitals
Before we dissect the individual orbital sketches, let's establish the overarching importance of these regions. Electron orbitals aren't just abstract concepts; they directly influence:
-
Chemical Bonding: The shapes and orientations of orbitals dictate how atoms interact and form bonds with each other. Overlapping orbitals facilitate the sharing or transfer of electrons, creating covalent and ionic bonds respectively. The specific type of bond formed is directly related to the orbital involved.
-
Molecular Geometry: The spatial arrangement of atoms within a molecule is a direct consequence of the arrangement of their constituent electron orbitals. This geometry significantly affects a molecule's physical and chemical properties.
-
Spectroscopic Properties: Electrons can absorb and emit electromagnetic radiation as they transition between different energy levels and orbitals. The wavelengths of these transitions are unique to each element and are the basis of spectroscopic techniques used for identification and analysis.
-
Reactivity: The availability of electrons in specific orbitals dictates an atom's or molecule's reactivity. Atoms with unpaired electrons in accessible orbitals are generally more reactive than those with filled orbitals.
Understanding Orbital Sketches: A Visual Representation
It’s crucial to remember that orbital sketches are simplified representations of complex three-dimensional probability distributions. They depict regions of high electron density – the areas where an electron is most likely to be found. The sketches don't show the electron's actual path, which is inherently unpredictable.
1. The Spherical s Orbital
The s orbital is the simplest type. Its sketch depicts a sphere centered on the nucleus. This doesn't mean the electron is confined to the surface of the sphere; rather, the probability of finding the electron decreases as you move further from the nucleus. The probability density is highest at the nucleus and gradually diminishes as you move outwards. All s orbitals are spherically symmetrical, regardless of the principal quantum number (n).
Key Features of the s orbital:
- Shape: Spherical
- Orientation: No specific orientation; spherically symmetric.
- Number of Nodes: An s orbital with principal quantum number n has (n-1) radial nodes. A 1s orbital has zero nodes, a 2s orbital has one radial node (a spherical surface where the probability of finding the electron is zero), and so on.
2. The Dumbbell-Shaped p Orbitals
The p orbitals are more complex than s orbitals. There are three degenerate p orbitals (meaning they have the same energy) within a given energy level: p<sub>x</sub>, p<sub>y</sub>, and p<sub>z</sub>. Their sketches often resemble dumbbells, with two lobes of electron density on either side of the nucleus.
Key Features of the p orbitals:
- Shape: Dumbbell-shaped, with two lobes of electron density.
- Orientation: Each p orbital is oriented along one of the three Cartesian axes (x, y, z). p<sub>x</sub> lies along the x-axis, p<sub>y</sub> along the y-axis, and p<sub>z</sub> along the z-axis. This directional nature is crucial for understanding chemical bonding.
- Number of Nodes: A p orbital has one nodal plane (a plane where the probability of finding the electron is zero). For p<sub>x</sub>, the nodal plane is the yz-plane, and similarly for p<sub>y</sub> and p<sub>z</sub>.
2.1. The p<sub>x</sub> Orbital
The p<sub>x</sub> orbital sketch shows a dumbbell shape aligned along the x-axis. One lobe of electron density is concentrated on the positive x-axis, and the other on the negative x-axis. The nucleus lies at the center, at the point where the lobes meet. The probability of finding the electron is zero at the nucleus itself and in the yz plane which forms a nodal plane.
2.2. The p<sub>y</sub> Orbital
The p<sub>y</sub> orbital sketch is identical in shape to the p<sub>x</sub> orbital but oriented along the y-axis. The lobes are situated on the positive and negative y-axis. The nodal plane is the xz-plane.
2.3. The p<sub>z</sub> Orbital
Similarly, the p<sub>z</sub> orbital has a dumbbell shape oriented along the z-axis, with lobes positioned on the positive and negative z-axis. The nodal plane for p<sub>z</sub> is the xy-plane.
3. Limitations of Orbital Sketches
It's crucial to understand that these sketches are highly simplified representations. They don't accurately reflect the nuances of electron behavior:
-
Probability Density: The sketches only represent regions of high probability. The electron's position isn't limited to the depicted lobes; it can exist anywhere in space, albeit with diminishing probability as you move further from the nucleus.
-
Three-Dimensionality: Representing three-dimensional objects on a two-dimensional page inevitably leads to loss of information. The depth and curvature of the orbitals are not fully captured in a simple sketch.
-
Quantum Mechanical Nature: Orbitals are fundamentally described by wave functions, which are mathematical expressions. The sketches are visual aids to understand these wave functions but do not fully capture their complexity.
Beyond s and p Orbitals: A Glimpse into d and f Orbitals
While s and p orbitals are the most commonly encountered in introductory chemistry, d and f orbitals also exist and play crucial roles in transition metal chemistry and lanthanide/actinide chemistry. Their shapes are far more complex than s and p orbitals, featuring multiple lobes and nodal planes.
-
d orbitals: There are five degenerate d orbitals, with complex shapes involving four lobes and two nodal planes (except for the dz² orbital).
-
f orbitals: Seven degenerate f orbitals have even more intricate shapes, with multiple lobes and nodal planes.
The complexity of d and f orbitals significantly influences the properties of transition metals and lanthanides/actinides, leading to their diverse and often striking chemical behavior.
Applications of Orbital Theory
The understanding of electron orbitals is not confined to theoretical discussions; it has practical applications in several fields:
-
Materials Science: Understanding orbital interactions is crucial for designing new materials with specific properties, such as conductivity, magnetism, or strength. Manipulating the electronic structure via orbital hybridization allows for tailoring materials to specific applications.
-
Catalysis: Many catalysts function by facilitating electron transfer between reactants. Orbital theory is essential to understanding the catalytic mechanism and designing efficient catalysts.
-
Drug Design: The shapes and interactions of molecular orbitals play a key role in drug-receptor interactions. Understanding these interactions helps in designing more effective and targeted drugs.
-
Spectroscopy: Spectral analysis heavily relies on electron transitions between orbitals. Orbital theory is essential for interpreting spectroscopic data and identifying molecules or atoms.
Conclusion: The Foundation of Chemical Understanding
The sketches of electron orbitals, while simplified, offer a fundamental visual representation of atomic structure. These orbitals, in their various shapes and orientations, aren't merely theoretical constructs; they are the basis for understanding chemical bonding, molecular geometry, reactivity, and a plethora of other important chemical and physical phenomena. Grasping the essence of these orbitals – their shapes, orientations, and the probability distributions they represent – is crucial for progressing in any field related to chemistry, physics, or materials science. The continued exploration and refinement of our understanding of electron orbitals will undoubtedly lead to advancements in many scientific and technological disciplines.
Latest Posts
Latest Posts
-
Which Patient Has The Lowest Risk For Developing Schizophrenia
Apr 02, 2025
-
Research Findings Indicate That Exercise Is Associated With
Apr 02, 2025
-
What Is The Theme Of The Book The Hobbit
Apr 02, 2025
-
Oration On The Dignity Of Man Summary
Apr 02, 2025
-
A Sailors Eligibility For Advancement To A Higher Paygrade
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Here Are Sketches Of Four Electron Orbitals . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
