Indicate Which Compounds Below Can Have Diastereomers And Which Cannot.
Onlines
Mar 25, 2025 · 5 min read
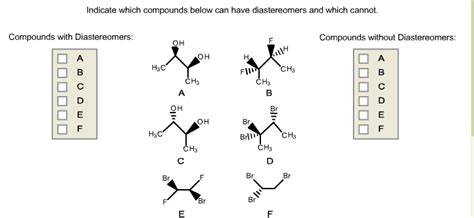
Table of Contents
Diastereomers: Identifying Compounds with and without the Possibility
Diastereomers are a fascinating aspect of stereochemistry, representing a specific type of stereoisomer. Understanding which compounds can exhibit diastereomerism and which cannot is crucial for organic chemists and anyone working with chiral molecules. This comprehensive guide will delve into the concept of diastereomers, exploring the necessary conditions for their existence and providing a detailed analysis of various compounds, clarifying which can and cannot have diastereomers.
What are Diastereomers?
Before diving into specific examples, let's establish a clear understanding of what diastereomers are. Diastereomers are stereoisomers that are not mirror images of each other and are not enantiomers. This means they possess chiral centers (stereocenters) but are not related as a pair of nonsuperimposable mirror images. Enantiomers are a special type of stereoisomer where the molecules are nonsuperimposable mirror images. Diastereomers, on the other hand, possess different configurations at one or more stereocenters but are not mirror images. This difference in configuration leads to different physical and chemical properties.
Conditions Necessary for Diastereomerism
For a compound to exhibit diastereomerism, it must meet the following criteria:
- Multiple chiral centers: The molecule must possess at least two chiral centers (stereocenters). A single chiral center only leads to the possibility of enantiomers, not diastereomers.
- Different configurations at at least one chiral center: The configurations at the chiral centers must be different in the diastereomers. If all chiral centers have the same configuration, the molecules are identical.
Identifying Compounds with Diastereomers
Let's examine various hypothetical compounds to determine which can and cannot have diastereomers. To illustrate, we will represent chiral centers using the wedge and dash notation.
Example 1: 2,3-Dibromobutane
2,3-Dibromobutane has two chiral centers (the second and third carbon atoms). Therefore, it can exhibit diastereomerism. The possible stereoisomers are:
- (2R,3R)-2,3-Dibromobutane
- (2S,3S)-2,3-Dibromobutane (Enantiomer of (2R,3R))
- (2R,3S)-2,3-Dibromobutane
- (2S,3R)-2,3-Dibromobutane (Enantiomer of (2R,3S))
The (2R,3R) and (2S,3S) are enantiomers. The (2R,3S) and (2S,3R) are also enantiomers. Importantly, the (2R,3R) and (2R,3S) are diastereomers, as are (2R,3R) and (2S,3R), and all other combinations where at least one chiral center has a different configuration.
Example 2: 2-Bromobutane
2-Bromobutane has only one chiral center (the second carbon atom). Therefore, it can only have enantiomers, not diastereomers. It exists as two enantiomers, (R)-2-bromobutane and (S)-2-bromobutane.
Example 3: 1,2-Dibromocyclohexane
1,2-Dibromocyclohexane, depending on the cis/trans isomerism, also exhibits diastereomerism. The cis isomer has only one possible structure. However, the trans isomer has two chiral centers and can exist as a pair of enantiomers. The cis and trans isomers themselves are diastereomers.
Example 4: Tartaric Acid
Tartaric acid is a classic example showcasing diastereomerism. It possesses two chiral centers, leading to three stereoisomers:
- (2R,3R)-Tartaric acid (D-tartaric acid)
- (2S,3S)-Tartaric acid (L-tartaric acid) (Enantiomer of D-tartaric acid)
- (2R,3S)-Tartaric acid (meso-tartaric acid)
(2R,3R)- and (2S,3S)-tartaric acid are enantiomers. (2R,3R)- and (2R,3S)-tartaric acid are diastereomers. Meso-tartaric acid is an achiral molecule despite containing chiral centers due to internal symmetry (a plane of symmetry).
Example 5: 1,4-Dibromobutane
1,4-Dibromobutane has no chiral centers. Therefore, it cannot have enantiomers or diastereomers.
Identifying Compounds Without Diastereomers
To lack diastereomers, a compound must either have no chiral centers or only one chiral center.
Compounds with No Chiral Centers: These compounds are achiral and cannot exhibit any type of stereoisomerism, including diastereomerism. Examples include: methane, ethane, and many symmetrical molecules.
Compounds with One Chiral Center: These compounds can only exist as a pair of enantiomers, which are non-superimposable mirror images of each other. Diastereomers require at least two chiral centers with different configurations. Examples include 2-bromopropane and 2-chlorobutane.
Advanced Considerations: Meso Compounds and More Complex Molecules
The presence of meso compounds adds another layer of complexity. Meso compounds are achiral molecules possessing chiral centers. This achirality arises from an internal plane of symmetry that cancels out the chiral effects of the individual centers. Meso compounds are diastereomers of other stereoisomers in the same set. For example, meso-tartaric acid is a diastereomer of both D- and L-tartaric acid.
As molecular complexity increases, so does the number of possible stereoisomers, including diastereomers. Molecules with multiple chiral centers and other elements of stereochemistry, such as cis-trans isomerism in alkenes or rings, can possess a vast number of diastereomers.
Practical Applications of Understanding Diastereomers
The ability to identify and differentiate diastereomers is vital in several areas:
- Drug Design and Development: Many pharmaceuticals are chiral molecules, and diastereomers often exhibit significantly different biological activities. Understanding diastereomerism is crucial for developing effective and safe drugs.
- Materials Science: Diastereomers can possess different physical properties, such as melting point, boiling point, and solubility, which are relevant for developing materials with specific characteristics.
- Natural Product Chemistry: Many naturally occurring compounds contain multiple chiral centers, and their diastereomeric forms often have distinct biological activities.
Conclusion
Identifying compounds capable of exhibiting diastereomerism involves a careful examination of molecular structure and the presence of multiple chiral centers with different configurations. Understanding this concept is fundamental to stereochemistry and has significant implications in various scientific fields. By systematically analyzing the structure of any molecule, you can effectively determine whether diastereomers are a possibility. Remember the key: at least two chiral centers with differing configurations are the essential condition for the existence of diastereomers. This article provides a comprehensive overview that can serve as a valuable resource for students and professionals alike. Remember to practice identifying chiral centers and determining the relative configurations (R/S) to master this fundamental concept of organic chemistry.
Latest Posts
Latest Posts
-
Summary Of Act 1 Scene 2 Of Julius Caesar
Mar 26, 2025
-
Ati Rn Evidence Based Practice Assessment
Mar 26, 2025
-
What Proportion Of Sales Occurred In The Northwest Region
Mar 26, 2025
-
Emerging Technologies In Cybersecurity C844
Mar 26, 2025
-
The Book Of The City Of Ladies Summary
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Indicate Which Compounds Below Can Have Diastereomers And Which Cannot. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
